B2 Rotavirus
B2.1 Introduction
-
Capacities taught in this chapter
Scientific processHow can people work together to find a vaccine against a major disease?Scientific toolsSurveillance: How widespread was rotavirus infection before the introduction of the vaccine?Reading and interpretingHow does the rotavirus enter a cell?Bridging science, society and the environmentWhy is scientific integrity and personal responsibility important for society?Quantitative skillsWhat is the epidemiology of rotavirus infections?
- gastroenteritis
- An infection in which the stomach and the intestine become inflamed, causing vomiting and/or diarrhoea.
In this chapter you will read about a virus that is not very well known, but one that is highly prevalent across the world and a leading cause of death in children younger than five years old in India: rotavirus. Rotaviruses are the most common cause of gastroenteritis in infants and young children. Diarrhoea and the consequent severe dehydration can lead to hospitalisation and sometimes causes death.
- enterocytes
- Cells that form the lining of the intestine.
Rotaviruses infect enterocytes within the intestine and cause malabsorption, which induces diarrhoea. Vomiting and fever are also associated with rotavirus infections.
Across the globe, people have been hearing about viruses due to the novel coronavirus SARS-CoV-2 pandemic. Although most viruses are completely harmless, it is extremely important to study viruses because they are infectious agents that can cause major, persistent illness in almost all life forms.
Our experience with the novel coronavirus has shown how important it is to understand various aspects of viruses: how they are transmitted to their hosts, how they cause disease, and how the immune system responds.
- cell theory
- The theory that all organisms are made of cells, that cells are the most basic unit of life, and that each cell is derived from a pre-existing cell.
The study of viruses forces us to ask a very fundamental question about what constitutes a living thing. Cells are often called the fundamental unit of life, as defined by the cell theory. This unit of life can reproduce and metabolise (process) substances.
Viruses are not cells and do not reproduce by cell division. Furthermore, viruses do not have their own metabolism: they rely on host cells to replicate their genomic material, as illustrated in Figure B2.1a. Yet, viruses do contain genomic material that stores information that will be maintained across many ‘generations’ or copies of the virus.

Figure B2.1a A typical viral cycle. The virus attaches to the cell and enters. Once it enters the cell, it releases its own genome into the host cell, and exploits the host’s machinery to replicate itself. How the virus’s genome interacts with the host depends on whether the virus is an RNA or a DNA virus. When the virus is released, the host cell is often lysed (burst) and destroyed.

Figure B2.1b A few typical viruses, which come in many shapes and sizes.
- horizontal/lateral gene transfer
- The transfer of genetic material from one cell to another, usually of a different species, and involving plasmids.
- vertical gene transfer
- The transfer of genetic material from a parent to its offspring.
- host-pathogen interaction
- The interaction that allows a pathogen to survive within its host.
From a more fundamental perspective, the study of viruses can give insight into many biological processes including horizontal gene transfer, vertical gene transfer, DNA/RNA replication, protein structure, and host pathogen interactions. In addition, biotechnologists have exploited viruses as tools for various applications from therapeutics to agriculture.
Viruses and disease
- virion
- A single virus particle consisting of the outer protein capsule and the inner genomic material (either RNA or DNA).
When a virus is outside a host cell, it exists as a free particle called a virion. Virions exhibit varied sizes and shapes (see Figure B2.1b), although most are smaller than 300 nm and cannot be seen with an optical microscope.
- capsid
- The protein shell that encapsulates a virus.
- protein-coding genes
- Genes that can be translated to mRNA, which is transcribed into proteins.
A virion is composed of either a DNA or an RNA genome that is enclosed in a protein shell called the capsid. The genome itself, whether DNA or RNA, may be double-stranded or single-stranded and may have varied arrangements. The number of protein-coding genes varies from 2 to 2000.
- host range (virus)
- The range of organisms that a virus can infect and in which it can replicate itself.
- phage
- A virus that injects its genome into a bacterial cell and uses the cell’s molecular machinery to synthesise more phages.
Large numbers of viruses exist on Earth, showing incredible diversity. Each virus is unique to a specific host range. They infect all living things, including plants, bacteria and humans. Phages are viruses that infect bacteria. The number of viruses on Earth is estimated at 1031, most of which infect bacteria.1
In humans, viruses cause widespread illnesses such as smallpox, dengue, chikungunya, measles, chicken pox and polio. India was declared free of polio in 2014 after a tireless national vaccination campaign that ran over nearly 40 years. Imagine running a vaccination programme for a country inhabited by more than a billion people!
The programme succeeded because of dedicated vaccination efforts and surveillance; successfully eliminating the disease is truly something worth celebrating. Read more about the history of polio eradication in India. The development of the first polio vaccine itself by Jonas Salk is an incredible story.
- virus strain
- A genetic variant of a virus that has slightly different characteristics from the original virus.
- zoonotic
- An infectious disease that has jumped from non-human animals to humans.
The human population is host to several strains of rotavirus. Every so often, patients present with uncommon or previously unidentified strains, which may come from animals. Many viruses, not just rotavirus, appear in humans through cross-species transmission from domestic or wild animals.2 The chances of zoonotic transmission increases as we clear away the habitat of wildlife and increase our interaction with previously wild species.
When a virus crosses the species barrier, it must evolve a mechanism to survive in a new host. Interspecies transmission is one of the important drivers of rotavirus evolution and increased diversity of human rotavirus strains. Scientists estimate that nearly 60% of emerging infectious diseases originate from zoonoses.3
- disease burden
- The effect of a disease in terms of morbidity, mortality, and financial cost to a population.
Low income countries suffer from a large disease burden due to rotaviruses.4 Every child in the world is infected by a rotavirus at least once. The widespread prevalence of rotavirus, and the economic burden associated with it, call for us to urgently address rotavirus infections in India, and globally.
In this chapter you will chart your way through viruses and their classification, and how scientists study clinically relevant viruses in the lab. You will learn how scientists use model systems such as monkeys, mice, flies, and even cells in culture for studies in the lab, since for ethical reasons it is not possible to experiment on humans.
You will see how science is a social enterprise that involves team collaboration and cooperation using the example of a large team of scientists working together to create the first indigenously developed vaccine. We will discuss why integrity and social responsibility play a vital role in reporting results of any scientific work.
Vaccines are developed for diseases that are either common problems such as rotavirus, which affects children all over the world, or diseases that occur as outbreaks that can disrupt society, such as cholera, or diseases for which there are few treatments and can be deadly. To decide whether a vaccine is needed, we must first understand the severity of the infection. Measuring incidence before a vaccine is developed or used allows for an estimation of disease burden. Once a vaccine is used, disease incidence can be monitored closely post-vaccination to measure the impact of the vaccination. We will read a paper that presents the surveillance programme that monitored the occurrence of rotavirus infections across India before the introduction of the vaccine. This reading will demonstrate how such data is reported and interpreted. Finally, you will have the opportunity to see and practise interpreting epidemiological data.
B2.2 Rotavirus: double-stranded RNA virus
Reading and interpreting
The name ‘rotavirus’ comes from the virus’s characteristic wheel-like appearance (Figure B2.2a). Its genome consists of 11 double-stranded RNA strands (Figure B2.2b). The genome codes for six viral proteins (VP) and six non-structural proteins (NSP).
Compare this number to more than 4000 protein-coding genes in the Escherichia coli bacterium, to 60 000 genes in Trichomonas vaginalis, which is a single-celled parasitic protist, and 25 000 genes in humans. Isn’t it surprising that such a small virus with so few genes has the ability to cause severe disease and even death in much larger animals with many more genes?
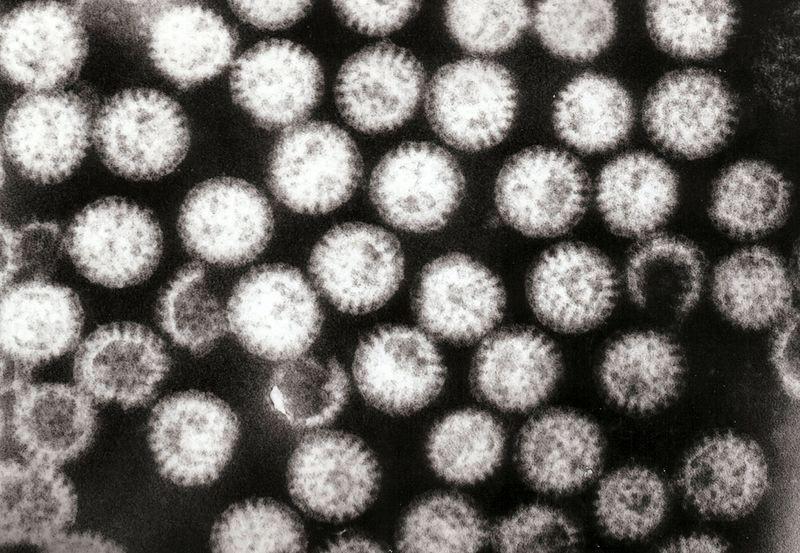
Figure B2.2a Transmission electron micrograph of rotavirus particles.
Graham Beards, Wikimedia, CC-BY 3.0

Figure B2.2b Diagram of virus with viral proteins (VP) and double-stranded RNA (dsRNA) genome.
Exercise B2.1 Rotavirus is an RNA virus
Reading and InterpretingQuantitative skillsThe rotavirus genome contains 11 segments of double-stranded RNA. Each of these segments codes for either non-structural proteins (NSP) or a viral protein (VP). Note that segment 11 codes for two proteins. These segment sizes in base pairs and the corresponding proteins they code for are given in the table below.
RNA segments 1 2 3 4 5 6 7 8 9 10 11 Protein VP1 VP2 VP3 VP4 NSP1 VP6 NSP3 NSP2 VP7 NSP4 NSP5 NSP6 No. of base pairs 3302 2690 2591 2362 1611 1356 1104 1059 1062 751 667 The number of RNA base pairs coding for each protein in the rotavirus.
- electrophoretic gel
- The gel used in gel electrophoresis. See also: gel electrophoresis.
Make a sketch of the 11 segments as they run on an electrophoretic gel and label each band (for more information, read the molecular biology primer). Recall that an electrophoretic gel, a polymer mesh, separates nucleic acids based on size by running an electric current through it. Since RNA is negatively charged, it will move towards the positive end, but the rate at which it moves depends on its size.
- faecal-oral
- A route of disease transmission which occurs through the ingestion of faecal particles.
The host takes in the virus through the faecal-oral route, or through contact with contaminated objects, or from other infected people, particularly children. Given the high prevalence of this virus, many people become reinfected over their lifetime.
Rotaviruses rarely affect adults because the disease becomes less severe with each reinfection. Previous infections lead to protection from the disease, but not from infection. In general, there is less protection for children in low income countries than those in rich countries. The precise reason for the difference is not known, but it may be because of more inflammation or poorer immune responses of children in these countries.
How does a rotavirus replicate inside a host cell and cause gastroenteritis?
How does rotavirus cause diarrhoea? The following paragraphs describe the virus-cell interaction. The information in these paragraphs is dense, so be patient, and see if you can visualise the processes that are described here.
- cell surface receptor
- Proteins spanning the cell membrane that can bind to extracellular molecules and conduct signals into the cell.
- endocytosis
- A process by which a substance outside a cell is taken into the cell.
The rotavirus enters the body through the gastrointestinal tract and attaches itself to a cell-surface receptor of an enterocyte. The cell membrane engulfs the virus and takes it into the cell via endocytosis.
- cytoplasm
- A thick solution that fills each cell.
Inside the cell, an endosome encapsulates the virus. Inside the endosome, the concentration of calcium is very low, which strips away the outer capsid coat of the triple-layered particle (TLP) or virus. The double-layered particle (DLP) which remains enters the cytoplasm of the cell.
Once in the cytoplasm, the DLP undergoes transcription to produce viral mRNA strands. Some of the transcribed mRNA is translated into proteins that will be used to assemble viruses, while the rest is used as a template for genome replication.
The RNA template, along with the translated proteins, enter a viroplasm, an area in the cytoplasm where virus assembly occurs. The replicated RNA is packaged into a fresh DLP. Another layer of proteins creates the third layer of the particle.
- lysed
- Broken (or burst) cell.
Once formed, this TLP enters the rough endoplasmic reticulum. Here the TLP undergoes budding and becomes a transient enveloped particle. Two outer capsid proteins attach and form the outer capsid coat. The envelope is lost while still in the endoplasmic reticulum and the resulting virion matures and is released into the environment through cell lysis, and moves on to repeat this cycle and infect other cells.
What happens when neighbouring cells become infected with the mature virus? How does this actually result in diarrhoea? The repeated cycle of infection results in the enterocytes on the upper surfaces of the villi in the intestine being destroyed. As the mature enterocytes die, younger cells come up to the top of the villi. These functionally immature cells are not able to absorb nutrients, which remain in the gut and draw water across the intestinal epithelium because of osmosis. In addition, rotavirus infection results in damage to the mechanisms by which sodium and chloride are transported in the gut so that water, instead of being retained, passes into the gut.
Exercise B2.2 Rotavirus enters the cell and replicates
Reading and interpretingIt is helpful to rework complicated written descriptions into simplified diagrams.
The description in the section entitled ‘How does a rotavirus replicate inside a host cell and cause diarrhoea?’ outlines the replication of the rotavirus inside the host cell.
Pair up with another student in your class or study circle and interpret this text and convert it into a diagram of the process. Then click on the answer to see if your interpretation shows the process in the correct order.
After you have compared your interpretation with the answer, try to compare your sketch with those created by other pairs in your class or study circle. What choice of colours are most popular among the different sketches? What are the shapes that have been used to depict different cell organelles or rotavirus? Why do you think this is? Find out what the true colours and shapes are.
Summary
By reading about the structure of rotavirus and its journey as it enters a host cell, you have had a chance to practise your reading and interpreting skills. Drawing diagrams of complicated sequential processes can be very helpful. Try to practise this skill the next time you read such text. Next we will look at how rotavirus is studied in the lab using a model system.
B2.3 How do we use model systems (including cell lines) to study Rotavirus?
Scientific process Scientific tools
- public health
- The study of the health of a population (community, town, city, state or country) as a whole.
As we have seen in the previous two sections, viruses can cause serious disease, and they do so by attacking certain cells of the body. To address the public health concern at the community and individual level, we need to gain a fundamental understanding of the disease at the cellular scale. The idea of gaining an understanding across length scales is addressed in the numbers and scales primer.
Model systems
How do we study disease at the molecular, cell and organismal scale? It is not ethical to subject a human patient to experimental investigations. What alternatives do scientists have?
You may have heard of drugs being tested on animals first before being tested in humans in pre-clinical trials. In this case, the animals (such as mice or monkeys) serve as models for humans. We first verify whether the drug functions the way we predict it will in a similar system, and then we conduct a trial on humans.
It is important to understand the idea of a model system or model organism because these are used for clinical trials and also for more fundamental scientific investigations. We use the term ‘model’ because it is not a replica, but a substitute that is similar to the ‘real’ thing (in this case the real thing is a human).
Scientists use a wide variety of model systems for applied and fundamental research. These include worms, flies, bacteria, yeast, algae, rabbits, mice and monkeys (see Figure B2.3).
Many discoveries in cell and developmental biology have been made using only a handful of model organisms. Here are a few examples of major discoveries:
- apoptosis
- Programmed cell death.
- Thomas Morgan Hunt used the common fruitfly (Drosophila melanogaster) in his seminal work on sex-linked inheritance.
- A roundworm (Caenorhabditis elegans) has been used extensively to study the process of programmed cell death, apoptosis.
- Baker’s yeast (Saccharomyces cerevisiae) has helped us understand how and when cells divide.
- One particular species of bacteria (Escherichia coli) is probably the most important model organism to have been used in any cell biology lab. It has been the ‘workhorse’ for countless biotechnological applications.

Figure B2.3 Common model organisms: bacteria (Escherichia coli), yeast (Saccharomyces cerevisiae), green algae (Chlamydomonas), cell line (fibroblast), organoid (tissue of differentiating cells), fruitfly (Drosophila melanogaster), hydra (Hydra vulgaris), planaria (Dugesia tigrina), tardigrade, roundworm (Caenorhabditis elegans), maize (Zea mays), Arabidopsis (Arabidopsis thaliana), frog (Xenopus laevis), mouse (Mus musculus), zebrafish (Danio rerio).
How can bacteria and yeast (being unicellular) teach us anything about human biology? The life cycle of fruit flies is so different from mammals, how could it have been useful in understanding developmental mechanisms in humans?
The answer lies at the core of biology: all of life evolved from a common ancestor, and in all organisms, genes determine which traits pass from one generation to the next. The process of evolution and inheritance through genetic material allow us to use model organisms to conduct experimental investigations.
Since most multicellular organisms share similar metabolic pathways, they may react similarly to external input. But we must be careful in choosing the model organism, as not all systems can be used for all investigations. It would be absurd to use bacteria to study neuronal processes or cell differentiation and development of a multicellular organism. However, studying bacteria can teach us about transcription and translation, which are processes that occur in all living things. In fact, gene regulation and the genetic code were understood in bacteria first.
Recently many scientists have recommended that we expand the range of model organisms we use.5 For example, organisms such as planaria or axolotls, which have incredible regenerative capacities, may be useful in studying regeneration and stem cells. Looking at how neurons develop, communicate, and interact may be easier in organisms such as squid that have large neurons.
From these examples, we see that by using carefully chosen analogous (similar) systems, we can learn a lot about human biology. But how do we choose the right model system? In what ways should the model be similar to the ‘real’ thing? Should the model have similar shape, size, structure, behaviour, and/or genetic content?
Further considerations are feasibility and cost. Maintaining a live, continuously propagating population of any model organism requires infrastructure, money, and a lot of time. Although the use of invertebrates for research is not very strictly regulated (it is believed that invertebrates experience less pain), we must still take ethical considerations into account. How many flies or worms should we experiment on? Is there a way to minimise harm?
Cell lines as model systems
- cell line
- Cultures of animal cells that are grown in controlled conditions (usually in laboratories) for many generations.
Another model system that addresses several of the concerns mentioned above is cell lines. Under the right conditions, human and other mammalian cells grow in the lab. Some types of cells extracted from tissue divide readily when placed in a liquid medium that contains the right type of nutrients. Often these cells originate from tumours. A cell line is a culture of human cells that proliferate and can be maintained in the laboratory for an extended period.
The HeLa cell line, for example, originated from the cervical tumour of an African American woman named Henrietta Lacks. Her cells were the first to proliferate so well in lab conditions. HeLa cells are found in practically every lab in the world that grows human tissue cultures. Since their isolation in 1951, they are believed to have gone through so many generations that each lab’s stock of HeLa cells may no longer be classified as human because they have changed so much.6 The story of the HeLa cell line is full of ethical questions that you can read more about.
Since the first breakthrough in tissue culture with HeLa cells, hundreds of cell lines have been established from various tissues (brain, breast, muscle, skin and many others), and organisms (from humans, dogs, monkeys, rabbits, mice, and even fish).
Now that you have had an introduction to model organisms, and cell lines in particular, let us review a couple of examples of how the rotavirus has been studied in the lab using model systems.
How rotavirus enters the cell using fluorescent tags in a cell line
How do we know what factors allow the rotavirus to enter the cell? In a 2017 study, Li et al. investigated how rotaviruses attach and internalise into host cells.7
While scientists knew that the outer capsid protein VP4 was required for this process, they did not know how it mediated the process. The study identified all the host proteins that interact with VP4.
- cytoskeletal proteins
- Proteins in the cytoplasm of a cell that help maintain its structure, provide mobility, and carry out mechanical functions.
- actin
- An intracellular protein that forms microfilaments that have several functions, including in cell movement.
The analysis revealed that VP4 interacts with many cytoskeletal proteins, and that the strength of interaction was especially strong with drebrin, also known as DBN1, a human host protein. DBN1 associates with actin, a primary cytoskeleton filament that restricts viral entry.
The researchers wanted to see where VP4 and DBN1 interact in the cell. How can we identify the location of a protein when proteins are so small? Recall that proteins are on the length scale of nanometres, and are too small to be seen.
- fluorescent tag
- A fluorescent molecule that is attached to a cellular molecule to improve the visualisation of the molecule, usually through microscopy.
There are several ways to add a fluorescent ‘tag’ to a protein. The fluorescent tags are very bright, and can be viewed through a fluorescence microscope so that you can trace the protein’s location. The fluorescent tags glow when subjected to specific wavelengths of light. To visualise the location of two different proteins, scientists can use differently coloured tags for each protein.
- fibroblast
- A connective tissue cell that produces collagen and an extra-cellular matrix.
Looking at Figure B2.4, you will see that VP4 is tagged in green, DBN1 in red and actin is white. The overlay panel on the extreme right points to the bright spots where VP4 and DBN1 are colocalised, meaning that they are found in the same places in the cytoplasm of the cell. This study was conducted with HEK293 cells, a cell line derived from human embryonic kidney fibroblast cells.
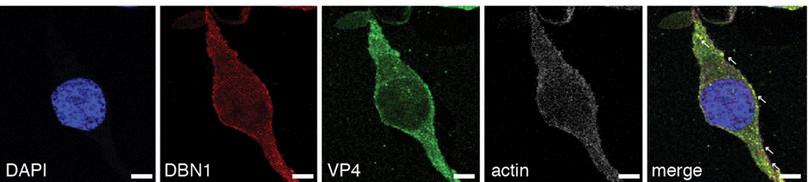
Figure B2.4 DBN1 interacts with actin. Note that the first image shows only the nucleus, the next three show the corresponding protein localisation in the cell through fluorescent tagging, and the final image shows the overlap of each protein. Arrows show positions where VP4 and DBN1 are colocalising.
Li, B et al., ‘Drebrin Restricts Rotavirus Entry by Inhibiting Dynamin-Mediated Endocytosis’, Proceedings of the National Academy of Sciences 114, no. 18 (2017): E3642–3651, doi: 10.1073/pnas.1619266114. PNAS exclusive License to publish for non-commercial and educational use.
What does this colocalisation indicate? It indicates that DBN1 interacts with VP4, and since the effect is to restrict viral protein entry, interruption of the interaction might increase entry.
Knockout experiments provided more evidence confirming this result. The researchers showed that if DBN1 is knocked out of the cell line (by repressing its expression), cell uptake of foreign toxins was enhanced and led to more damage to the host cell.
This result gives us insight into a crucial aspect of the rotavirus entry into the host. Using a cell line was crucial for the studies for several reasons. The two-dimensional (flat) nature of such tissue cultures allows us to visualise viral protein localisation within individual cells. Controlling gene expression is relatively easy in cell lines. Experiments with cell lines also tend to be much faster.
Organoids as model systems
- organoids
- Three-dimensional tissue cultures that are simplified versions of organs.
The intestine, the primary location of rotavirus infections, is not flat, and contains several different cell types. Organoids have been increasingly used as model systems for studying the issue of morphology and cell diversity.
Organoids are simplified and miniaturised versions of specialised tissue that are grown in the lab. They are often grown from human stem cells or engineered induced pluripotent stem (iPS) cells which are then induced to differentiate into the desired cell type. Organoids are three-dimensional structures, mimicking many desired aspects of the target tissue, including genetic makeup.
- host specificity
- A life history trait of a pathogen that determines the number of host species it can inhabit or infect.
Human rotaviruses do not easily infect most regular animal cell line models, making laboratory studies difficult. In the next study, Saxena et al. used an organoid called a human intestinal enteroid (HIO or HIE) to test host specificity.8 An HIO is a three-dimensional organoid composed of many types of intestinal epithelial cells.
- in vivo
- Processes that occur within a living cell or organism.
Intestinal biopsy specimens from adult patients were used to establish, grow and further differentiate the HIOs in the laboratory. As shown in Figure B2.5, the HIOs developed by Saxena et al. were actually susceptible to infection by human rotaviruses but not to animal rotaviruses. This experiment demonstrated that the HIOs recapitulate the host specificity seen in vivo.

Figure B2.5 Percentage of cells infected with a human rotavirus strain (HRV), an animal rotavirus strain (ARV), and a mock (placebo).
Data from Saxena, K et al., ‘Human Intestinal Enteroids: A New Model To Study Human Rotavirus Infection, Host Restriction, and Pathophysiology’, Journal of Virology 90, no. 1 (2015): 43–56, doi: 10.1128/JVI.01930-15.
Summary
In this section, we highlighted the advantages and limitations of using a model system. Several ethical questions arose, from the use of cell lines, to the idea of minimising harm to all life. We also learned about how cell lines and organoids can be used to study details of how viruses enter the cell and host specificity.
B2.4 Team work: team science in developing the vaccine
Scientific process Reading and interpreting
Global inequality in disease burden
Disease burden is often disproportionately high in low income countries. The reasons for this can include pollution, malnutrition, and insufficient access to sanitation, health education and medical facilities. However, diseases occurring in geographic areas in the Global North receive more attention from medical and pharmaceutical communities. This is because the economic benefit of producing drugs for diseases found in high income countries is often far greater than in low income countries.
- absolute burden
- Cumulative consequence of disease.
Neglected tropical diseases (NTDs), including illnesses caused by bacterial, parasitic, viral, and fungal infections, are a prime example of this inequality. India experiences the largest absolute burden of several NTDs, including hookworm infections, leprosy and rabies.9 Although rotaviruses are found worldwide, in many places rotaviral infections peak during the winter months, whereas in tropical countries they occur throughout the year.10
The risk of reinfection, the most susceptible age group, vaccine efficiency (or efficacy) and the distribution of rotavirus strains all vary geographically. Finding local solutions through regional and national level cooperation could therefore contribute to solving local problems.
Team work in solving health problems and vaccine development
Solving public problems such as health, waste management, pollution, and health education involves people from many fields as well as active participation from citizens. Working in groups is a fundamental aspect of the scientific endeavour, whether it is for fundamental or applied research.
Vaccine development is a lengthy process, typically requiring a decade. It is much like developing a drug: researchers must find a candidate vaccine which must pass three phases of clinical trials and must carry out long-term surveillance to find and test all children when developing vaccine programmes. The candidate vaccine must meet regulatory requirements and acquire financial investment.
Because vaccines require so many resources to develop, they are often developed by resource-rich, profit-geared pharmaceutical companies. Once a vaccine is approved, sales must cover the costs of development and make a profit for the company. Most companies offer drugs and vaccines at discounted rates or even free to poor countries, but these offers are usually for limited periods of time.
Recent global experiences with the coronavirus have shown that vaccines can be developed very quickly if necessary. Of course, this kind of rapid development and testing required concerted effort and investment. In the case of the coronavirus vaccines, efforts have been led and supported by a large portion of the international community. It is an important example that shows that we need not take any established scientific processes for granted, and that there may always be room for improvement!
- attenuated
- Having a reduced effect; for example viruses that have been treated to reduce their virulence/harmfulness.
Indian children have, since 2008, been able to choose from two live attenuated vaccines against rotavirus. The vaccines available in private practice are Rotarix (GSK Biologicals, Rixensart, Belgium) and RotaTeq (Merck and Co., Pennsylvania, USA).
- Universal Immunisation Programme
- A public health programme developed by the government of India to provide free vaccination and immunisation to pregnant women and children against diseases such as polio, rubella, diphtheria, and others.
The vaccines have a much higher efficiency in high income countries than in India. In the USA, the vaccine dramatically decreased the rate of rotavirus-related hospitalisation.4 However, in India, the cost per dose was nearly $15 (that is nearly 1100 rupees) making the vaccine inaccessible for most of the population. It was impossible to include either vaccine in the Universal Immunisation Programme (UIP) for all children in India.
A locally developed vaccine for India
In 2016, India introduced an indigenously developed vaccine against rotavirus into the UIP. The vaccine is based on an Indian strain, discovered by a group of Indian scientists, supported by the global community. The development of this vaccine (summarised in Figure B2.6) is a rare and highly fruitful example of team science drawing on expertise from medical doctors, scientists, and governmental and non-governmental organisations.11
The story begins with a paediatrician at the All India Institute of Medical Sciences (AIIMS) in Delhi. With the help of research funding from the Department of Science and Technology, in 1988, Dr. Bhan and his team found a strain of rotavirus that was infecting newborns but did not result in illness.
This discovery was the catalyst for further research conducted by scientists at the Centers for Disease Control and Prevention, Atlanta, and Stanford University, USA. The initial phase involved an assessment of the immune response to the newly discovered strain. In 1988, government funding from the USA–India Vaccine Programme, the National Institutes of Health (USA), and the Department of Biotechnology in India, made Phase I clinical trials possible. Stakeholders from different countries were involved, requiring a tremendous amount of communication, careful documentation, and cooperation across continents.
By 2000, several organisations were providing skills and funding, including the Bill and Melinda Gates Foundation, PATH, AIIMS, and the Translational Health Science and Technology Institute (THSTI). It took nearly a decade to complete pilot tests and Phase I and II clinical trials to finally yield a candidate vaccine for Phase III trials.
- formulation
- A process in production of vaccines that involves the combination of adjuvants and antigens to optimise an immune response and ensure safety of the vaccine. See also: adjuvant.
The Phase III clinical trial started in 2008, and was conducted at multiple centres: SAS (Delhi), CMC (Vellore), and KEM Hospital Research Centre (Pune). The project moved to a public-private partnership modality, and an industry partner, Bharat Biotech, was identified to help with the formulation. Nearly 7000 children were enrolled in the clinical trials across these three centres.
In 2013, the results of the Phase III trial showed that the vaccine prevented 55% of severe rotavirus gastroenteritis, which made it comparable to Rotarix and RotaTeq, which had a similar performance in Africa, although they did much better in high income countries. In 2014, India’s National Technical Advisory Group on Immunisation recommended that the vaccine be introduced into the Universal Immunisation Programme, but recommended further surveillance after the introduction of the vaccine.

Figure B2.6 Flow chart highlighting key milestones of ROTAVAC development.
Remember that a clinical trial is a complex process, requiring careful documentation of how the vaccine is administered, and tracking many parameters including potential side effects, long-term immunity, and so on. ROTAVAC® was approved and first introduced into the UIP in four states in 2016, five additional states in 2017, and gradually phased into all remaining states in India.
Dr Gagandeep Kang narrates the processes that were involved in developing India’s rotavirus vaccine.
- intussusception
- A condition in which a part of the intestine slides into an adjacent part of the intestine, causing a telescopic effect which leads to blockage of the intestine.
One of the major risks associated with rotavirus infections and rotavirus vaccines is intussusception. Intussusception occurs when a portion of the intestine slides into an adjacent part of the intestine, in a telescoping effect that results in a bowel obstruction that can often be fatal.
The exact cause of intussusception and the reason for a slightly increased incidence after administration of a rotavirus vaccine is not known. Nevertheless, given this elevated incidence, it is essential to monitor intussusception along with any other side effects caused by the vaccine.
As recommended by the National Technical Advisory Group on Immunisation, once the vaccine was introduced, a long-term surveillance programme was established in 2018 across a network of 28 hospitals (locations shown in Figure B2.7) to identify cases of paediatric intussusception.12 and to measure the impact of the vaccine introduction. After examining collection information for almost four years, it was shown that the Indian rotavirus vaccine did not cause intussusception in Indian children, a finding similar to Rotarix in Africa, but different from Rotarix and Rotateq in high income countries where a small increased risk has been shown with both vaccines. A study to find out how much the vaccine reduces diarrhoea requires the collection of stool samples from children and testing them for rotavirus. This study is ongoing.
Over many years, researchers will collect thousands of samples. The surveillance programme has to ensure that every sample is collected and analysed under the right conditions, data is documented, and the results are gathered in a central location.
Personnel must receive proper training across the whole network of hospitals. The personnel must understand the importance of data integrity. If they make mistakes with data collection, it will influence the outcome of the impact and safety review of the vaccine, ultimately affecting every child in the country who will receive the vaccine as part of the UIP.
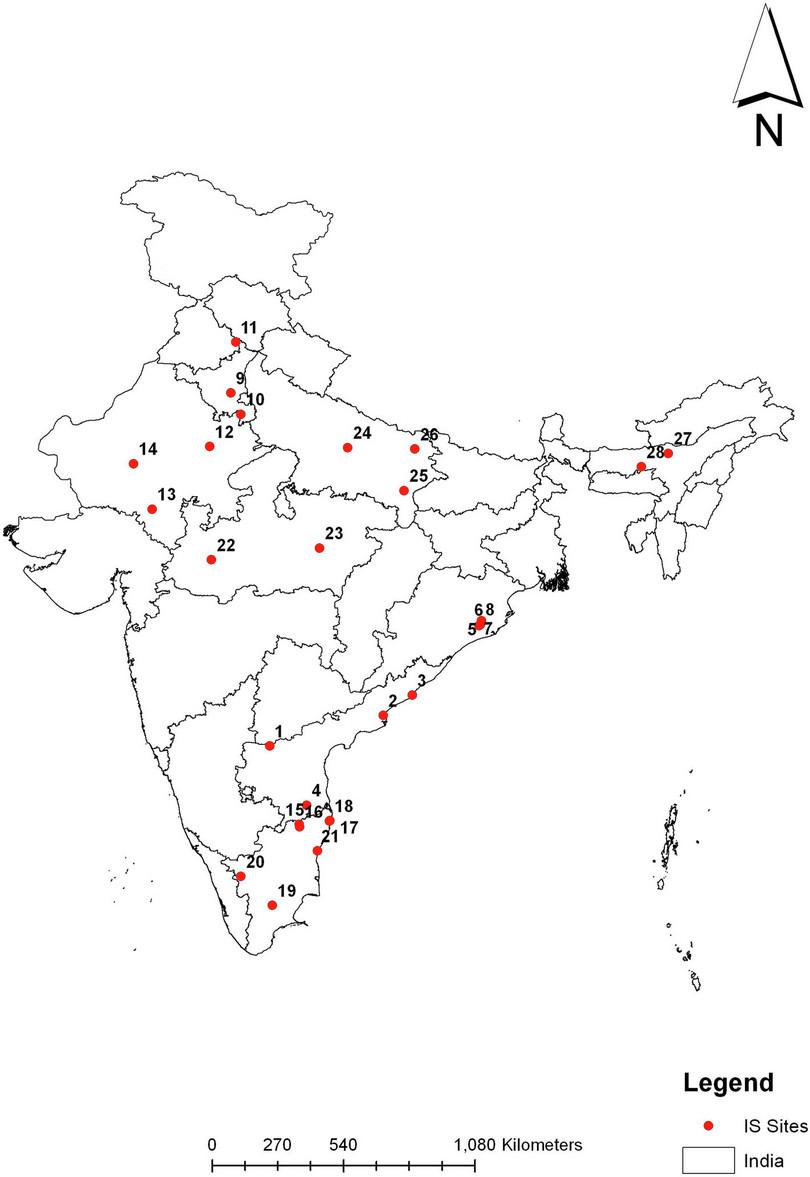
Figure B2.7 Vaccination surveillance sites in India.
Reddy, S et al., ‘Safety Monitoring of ROTAVAC Vaccine and Etiological Investigation of Intussusception in India: Study Protocol’, BMC Public Health 18, no. 898 (2018): 1–8, doi: 10.1186/s12889-018-5809-7, CC-BY 4.0
Data integrity and public responsibility
As you read through this course material, we hope you will see how intricately linked the practice of science is to society and the environment. If scientists are not honest in reporting their findings, what kinds of consequences could there be?
- scientific process
- Process of gaining knowledge through observation, hypothesis formulation, hypothesis testing, analysis of obtained data, and inference from analysis.
Firstly, you should appreciate that every bit of information we learn from the scientific process is dependent on somebody else’s work. This means that if the previous work was erroneously reported, our work too is likely to be invalid. If, for example, scientists had not been responsible in accurately reporting their findings on how rotavirus impacts the immune system, the development of the vaccine could have been severely impaired. Therefore, being honest and careful in recording and reporting any data is absolutely essential. Note that we use the word careful here as well, because a sloppy and negligent attitude towards data integrity is equally dangerous.
In most instances, scientific work is publicly funded (by taxpayers). Scientists therefore have a moral obligation to communicate their work properly to the public.
But what about smaller experiments? Suppose you were conducting a study in your school or college lab. How important is it to be honest about your results there? If you think deeply about this question, you will see that this too has implications far beyond your own project report. Students who come after you will often build on the work that you have done, so their success depends on yours. Your work sets an example for future batches of students and scientists.
Finally, when any member of the public finds out about instances of dishonesty or fraud in the scientific community, it significantly undermines the public’s confidence in the scientific endeavour. This in turn has an impact on how science is funded, and consequently on the kinds of innovations and discoveries that can be made. You may be interested in reading a case study about a scientist who claimed to show a link between autism and the measles vaccine.13 His work increased public skepticism about vaccines. Such vaccine hesitancy is believed to have led to several measles outbreaks.14 His results were later widely discredited due to several ethical violations and misrepresentations. The cost of this lack of scientific responsibility has been great.
Summary
What do you take away from the story of ROTAVAC? The story has demonstrated the importance of collaboration and leveraging the expertise of people from varied backgrounds. The impact of our work as scientists and the significance of honest recording and reporting was also highlighted.
Research Highlight Pre-vaccination surveillance
If a vaccine successfully reaches a clinic after being thoroughly tested for safety and efficacy, how do we assess the success of the subsequent vaccination programme? What should the measures of success be? Should we expect to see a complete eradication of rotavirus-derived illnesses (as we have seen in the case of smallpox), or an overall reduction in the number of cases? What percentage reduction would be enough for us to call the vaccination programme a success?
Rotavirus has been classified into 10 different species (A–J), which are themselves subclassified into various types based on the genotype. It is therefore important to identify whether there is a diversity in the response to different types of rotavirus.
To answer any of these questions it is important to have a baseline quantitative measure of the incidence of illnesses and deaths associated with any particular rotavirus types before the vaccine is introduced. This is called pre-vaccination surveillance. Once we have baseline data, we can evaluate the efficacy of the vaccine. A pre-vaccination surveillance was conducted between 2005 and 2016 in a multi-centric study of the incidence of rotavirus.15
This study tested nearly 30 000 stool samples for rotavirus infection from 28 sites across India. Each sample was tested for the presence of rotaviral infection. The genotypes of a subset of these samples was also determined to identify the rotavirus strain. The study essentially measured the disease burden of rotavirus before the introduction of the vaccine. We will study the publication that reports on the results of this surveillance study.
DownloadDownload annotated paperOnce you have read through this paper, you will be better equipped to analyse epidemiological data in the next section.
B2.5 Interpreting epidemiological data based on rotavirus
Reading and interpreting Quantitative skills
The ability to interpret quantitative data is an important skill in our everyday lives (both as a member of society, and in particular as scientists). In public health and epidemiology, these skills are particularly important. Interpreting such data will inform how we, as a society, decide to address any threats or risks to public health.
To investigate an example, study the pyramid graph in Figure B2.8 and answer the questions in Exercise B2.3.

Figure B2.8 Events and risk related to rotavirus in Indian children under five years old.
Adapted from John J, Sarkar R, Muliyil J, Bhandari N, Bhan MK and Kang G, ‘Rotavirus Gastroenteritis in India, 2011–2013: Revised Estimates of Disease Burden and Potential Impact of Vaccines’, Vaccine, 32, Suppl. 1 (2014): A5–A9. doi: 10.1016/j.vaccine.2014.03.004, PMID: 25091681.
Exercise B2.3 Events and Risk related to rotavirus
Reading and interpretingQuantitative skills
- The risk of children <5 years dying due to rotavirus in India is 1 out of 345 children. Which part of the diagram relays this information?
- What is the risk of hospitalisation due to rotavirus for children under five?
- How many children under five will go to the hospital for an outpatient visit due to rotavirus?
- Calculate the risk of an outpatient visit as a percentage. Write your answer in the form of a statement.
- Is the risk of hospitalisation due to rotavirus higher than death? Give a reason for your answer.
Geographic distribution of rotavirus
Another important aspect of public health is to look at how the disease is distributed. Figure B2.9 illustrates the number of deaths and the rates of mortality due to rotavirus in two separate maps.
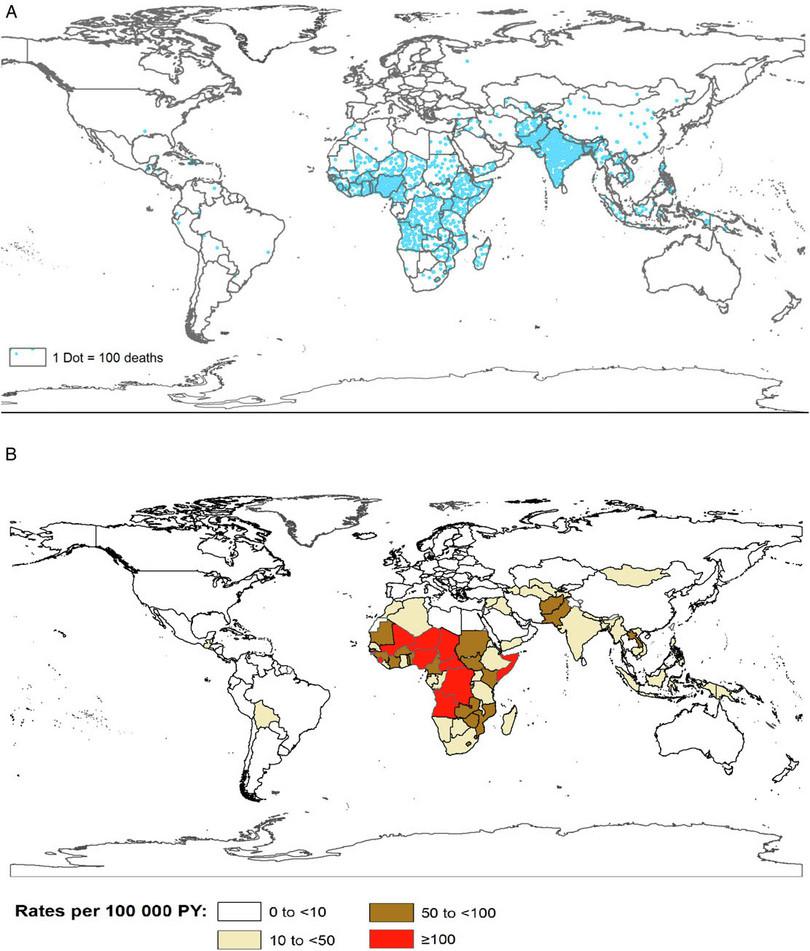
Figure B2.9 a. Number of rotavirus deaths in 2013. Each dot represents 100 deaths. b. Rate of rotavirus deaths globally in 2013 given in person years (PY). PY is a statistic used for disease incidence. For example, if a study followed 1000 people for one year, it would contain 1000 person years of data.
Tate, JE, Burton, AH, Boschi-Pinto, C and Parashar, UD, ‘Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013’, Clinical Infectious Diseases 62, Suppl. 2 (2016): S96–105, doi: 10.1093/cid/civ1013. Public domain.
Exercise B2.4 Disease distribution and incidence
Reading and interpretingQuantitative skills
- Which parts of the world have the highest and lowest number of deaths due to rotavirus?
- Explain why the rate of mortality (Figure B2.9b) is a more representative statistic than raw numbers (Figure 2.9a).
- Which parts of the world have the highest and lowest rate of death due to rotavirus?
What is herd immunity?
- herd immunity
- Protection achieved by a population when a large proportion of that population gains immunity to a disease, either through infection or vaccination.
Herd immunity is an epidemiological term used to describe the situation when a large proportion of the population becomes immune to a disease. The likelihood of the infection passing on to the small proportion who are not immune is greatly reduced. Herd immunity is therefore desirable.
Widespread infection can lead to herd immunity, however some diseases carry a high mortality risk or the risk of lifelong disability. Exposing many individuals to the infection and allowing them to fall sick contributes to herd immunity, but it is highly risky and very unethical.
The safer way to build herd immunity is through vaccination. For more details on how a vaccine works, visit the chapter on Malaria. Vaccinations are a safe and effective way of building herd immunity without risking infection. While the vaccine exposes your body’s immune system to the pathogen, you do not get ill because the pathogen has been weakened. If enough people in a population are vaccinated, the risk of a disease outbreak is greatly reduced. Both the effectiveness of the vaccine and how easily the disease gets transmitted influence herd immunity.
What percentage of a population must be vaccinated or survive a disease to achieve herd immunity? What factors influence the calculation of herd immunity?
The threshold for herd immunity varies with the disease. For example, for polio the threshold is around 80%, in other words, 80% of the population must have been vaccinated or had the disease. For H1N1 (commonly known as swine flu) the threshold is around 40%.16 New diseases or new strains of a disease make it difficult to calculate herd immunity because we do not know how easily such a disease spreads in a population.
Exercise B2.5 Herd immunity
Reading and interpretingQuantitative skillsScientists use the formula \(R=R_0 \times(1-P)\) to calculate the herd immunity percentage threshold.16
- R is the effective reproductive number, which is the average number of uninfected people in a partially susceptible population that can get infected. This provides a realistic scenario in which not everyone is vulnerable to the disease. This number must be below 1 in order to achieve herd immunity. If an infected person can only transmit the infection to one other person, an outbreak of the disease can be prevented.
- R0 is the basic reproductive number, which is the average number of uninfected people in a completely susceptible population to whom an infected person can transmit the infection.
- P is the percentage of the population that is immune to the infection.
If the threshold for a disease is set at 86%, what would the R0 value be?
The life span of herd immunity
Does herd immunity last forever? This depends on several factors. If there are still pockets of the population who carry the virus, and immunity for the disease wears off quickly, an outbreak may occur again.
Chicken pox is an interesting example. Children transmit chicken pox easily and it is not very dangerous to children but it is dangerous in adults. The virus remains in the population, but since immunity can last a lifetime, it does not pose a serious threat. This is why many parents expose their children to chicken pox (through ‘chicken pox parties’) early in life. But one cannot foretell the consequences of contracting chickenpox, some children may have grievous effects. Hence, it is recommended to vaccinate your children against chickenpox as it is much safer than the unpredictable consequences of infection.
As mentioned above, reinfection with the rotavirus causes very few symptoms. The mechanism of this increased immunity in rotavirus is unknown. Recent evidence indicates that some high income countries are likely to have achieved a high proportion of population immunity, and it is likely that vaccinations have contributed to this immunity. Because children are protected through vaccination, they get sick less frequently, shed the virus less frequently, and this results in fewer cases overall.
Summary
In this section you had a chance to practise your quantitative skills by dealing with epidemiological concepts and data of different kinds. Remember that interpreting quantitative data in biological systems must be done carefully, as this analysis can often influence public policy.
B2.6 Quiz
Question B2.1 Choose the correct answer(s)
Which of the following is true of viruses?
- Viral genomes can be single-stranded or double-stranded RNA or DNA.
- Viruses can cross species barriers when mutations occur that allow them to infect and replicate in a different host.
- With an estimated 1031 viruses on earth, most are completely harmless.
- Viruses can cause cancer, and the human papillomavirus is one such example.
Question B2.2 Choose the correct answer(s)
Which of these statements about model organisms is true?
- Organoids are three-dimensional, so it may be easier to visualise protein localisation in a two-dimensional model.
- Cell lines consist of a single cell type so signalling between cell types will not take place.
- Axolotls do in fact have incredible regenerative properties.
- Since mango trees can take several years to fruit, it would be more appropriate to choose a plant that fruits more quickly.
Question B2.3 Choose the correct answer(s)

Schematic of a hypothetical fluorescence image of a cell with a. protein labelled in green, b. filamentous protein labeled in blue, c. proteins in the nucleoplasm labelled in red and d. the overlay of all images.
By looking at this figure, identify which of the followings statements are true:
- The overlay image does not show an overlap of red and blue.
- The overlay image does not show an overlap of red and blue.
- The overlay image shows an overlap of green and blue and also green and red (note the yellow where green and red overlap).
- The overlay image shows an overlap of green and blue and also green and red (note the yellow where green and red overlap).
Question B2.4 Choose the correct answer(s)
‘Rotavirus disease … occurs at a younger age in low-income countries than in high-income countries … The proportion of all rotavirus hospitalizations in children <5 years of age that occur in infants by 8 months of age is 43% in Africa but only 27% in Europe.’4 Based on this quote from a research article, choose which of the following statements is true.
- The quote does not give absolute numbers to confirm this statement.
- The quote gives the proportion of infants by eight months of age out of the total number of hospitalizations for children under five hospitalised for rotavirus.
- The quote does not give statistics for adults.
- The quote does not give information about healthcare provision.
Question B2.5 Choose the correct answer(s)
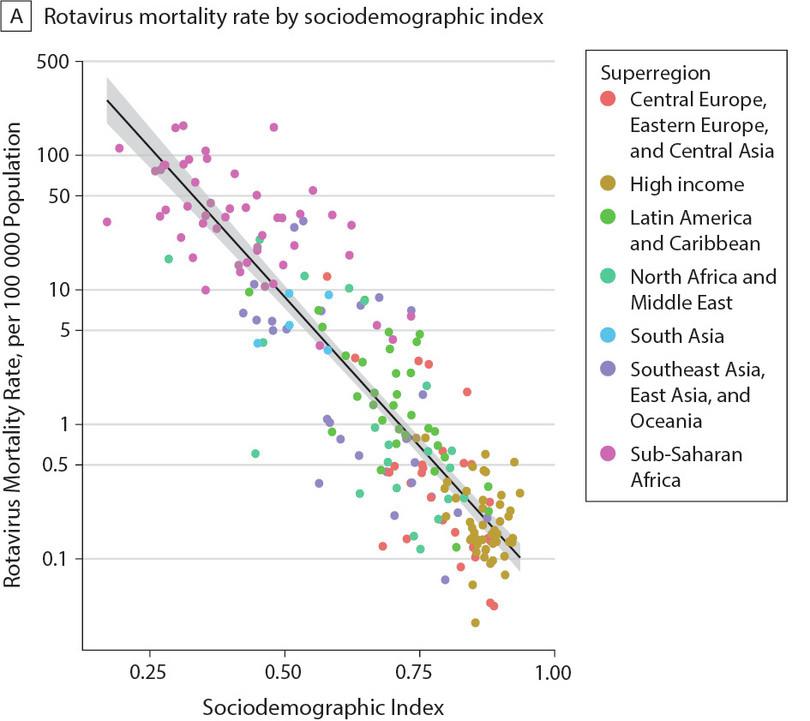
Global rotavirus mortality as a function of sociodemographic index. Each data point corresponds to a country in the given region.
Troeger, C et al., ‘Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years’, JAMA Pediatrics 172, no. 10 (2018): 958–965, doi: 10.1001/jamapediatrics.2018.1960. CC-BY
A sociodemographic index is one measure of how developed a country is. It ranges from 0 to 1, with 0 being undeveloped and 1 being fully developed. This index is calculated based on many different social, economic and demographic factors like per capita income, fertility rate, average level of education obtained, and so on.
Use this figure to choose which of the following statements are true.
- This data does not say anything about cause. We only see that there is a strong correlation between the sociodemographic index and rotavirus mortality.
- You can this is true by looking at the cluster of data points at the upper left part of the plot all corresponding to sub-Saharan Africa.
- You can see that as sociodemographic index increases, rotavirus mortality decreases, hence the negative correlation.
- The sociodemographic index is not a measure of rotavirus survival.
B2.7 References
-
Breitbart, M and Rohwer, F, ‘Here a Virus, There a Virus, Everywhere the Same Virus?’, Trends in Microbiology 13, no. 6 (2005): 278–284, doi: 10.1016/j.tim.2005.04.003. ↩
-
Cook, N, Bridger, J, Kendall, K, Gomara, MI, El-Attar, L and Gray, J, ‘The Zoonotic Potential of Rotavirus’, The Journal of Infection 48, no. 4 (2004): 289–302, doi: 10.1016/j.jinf.2004.01.018. ↩
-
World Health Organization, Zoonotic Disease: Emerging Public Health Threats in the Region, accessed 31 December 2020. ↩
-
Crawford, SE et al., ‘Rotavirus Infection’, Nature Reviews Disease Primers 3, no. 17083 (2017): 1–16, doi: 10.1038/nrdp.2017.83. ↩ ↩2 ↩3
-
Singer, E, ‘Biologists Search for New Model Organisms’, Quanta Magazine, 26 July 2016, accessed 31 December 2020. ↩
-
Oliwenstein, L, ‘No Longer Human’, Discover Magazine, 1 December 1992, accessed 24 December 2020. ↩
-
Li, B et al., ‘Drebrin Restricts Rotavirus Entry by Inhibiting Dynamin-Mediated Endocytosis’, Proceedings of the National Academy of Sciences 114, no. 18 (2017): E3642–3651, doi: 10.1073/pnas.1619266114. ↩
-
Saxena, K et al., ‘Human Intestinal Enteroids: A New Model To Study Human Rotavirus Infection, Host Restriction, and Pathophysiology’, Journal of Virology 90, no. 1 (2015): 43–56, doi: 10.1128/JVI.01930-15. ↩
-
Hotez, PJ and Damania, A, ‘India’s Neglected Tropical Diseases’, PLOS Neglected Tropical Diseases 12, no. 3 (2018): e0006038, doi: 10.1371/journal.pntd.0006038. ↩
-
World Health Organization, Rotavirus, WHO Vaccine-Preventable Diseases Surveillance Standards (2018), accessed 5 November 2021. ↩
-
Bhan, MK et al., ‘Team Science and the Creation of a Novel Rotavirus Vaccine in India: A New Framework for Vaccine Development’, The Lancet 383, no. 9935 (2014): 2180–2183, doi: 10.1016/S0140-6736(14)60191-4. ↩
-
Reddy, S et al., ‘Safety Monitoring of ROTAVAC Vaccine and Etiological Investigation of Intussusception in India: Study Protocol’, BMC Public Health 18, no. 898 (2018): 1–8, doi: 10.1186/s12889-018-5809-7. ↩
-
Rao, TSS and Andrade, C, ‘The MMR Vaccine and Autism: Sensation, Refutation, Retraction, and Fraud’, Indian Journal of Psychiatry 53, no. 2 (2011): 95–96, doi: 10.4103/0019-5545.82529. ↩
-
Gardner, L, Dong, E, Khan, K and Sarkar, S, ‘Persistence of US Measles Risk Due to Vaccine Hesitancy and Outbreaks Abroad’, The Lancet Infectious Diseases 20, no. 10 (2020): 1114–1115, doi: 10.1016/S1473-3099(20)30522-3. ↩
-
Giri, S et al., ‘Diversity of Rotavirus Genotypes Circulating in Children < 5 Years of Age Hospitalized for Acute Gastroenteritis in India from 2005 to 2016: Analysis of Temporal and Regional Genotype Variation’, BMC Infectious Diseases 20, no. 1 (2020): 740, doi: 10.1186/s12879-020-05448-y. ↩
-
Smith, PG, ‘Concepts of Herd Protection and Immunity’, Procedia in Vaccinology 2, no. 2 (2010): 134–139, doi: 10.1016/j.provac.2010.07.005. ↩ ↩2