D3 The kingdom of fungi
D3.1 Introduction
-
Capacities taught in this chapter
Reading and interpretingHow do fungi grow? What are some of the ecological functions they carry out?
What impact do fungal diseases have on agricultural plants?Scientific processHow do we characterise fungal biodiversity?Scientific toolsHow can we determine if a particular location has more fungal species?Bridging science, society and the environmentCan mushroom cultivation provide food security?
Can we harness indigenous knowledge of edible mushrooms and their medicinal properties?Quantitative skillsHow can we identify bioactive compounds from fungi?
The kingdom of fungi comprises a vast set of species with diverse morphologies and life cycles. Conservatively, the number of fungal species has been estimated to be 1.5 million.1 When compared to the kingdoms of plants and animals, fungi are poorly studied. It has been suggested that over 90% of fungal species remain uncharacterised. In fact, in the year 2019, 61 new fungal species were described in India.2
Think, for a moment, about fungi. What is the first thing that comes to your mind? Chances are that you might think of edible fungi such as mushrooms. If you have done some baking, you may think of yeast. You may also have seen mould growing on bread or fruit.
Fungi are found everywhere. You might be surprised to read that scientists have isolated previously undescribed fungal species from the screens of smartphones.
Mushrooms, moulds and yeast represent a small fraction of fungal diversity. Doing justice to this diversity is beyond the scope of this chapter. However, you will get glimpses of various aspects of fungal biology. You will see that fungi perform important ecological functions and are also a source for useful bioactive compounds such as antibiotics.
You might be wondering why fungi receive less attention than other organisms. It is a mistake to think that studying fungi is less important than studying plants or animals. There are several factors at play here.
- Most fungi (and fungal structures) are not visible to the naked eye.
- Since many fungi are small and immotile, we tend to assume that they do not carry out important functions and that they cannot exhibit interesting behaviours. This perception is rapidly changing as scientists describe the interactions between fungi and other species. Several fungi are used as model systems in biology (including baker’s yeast).
In this chapter, we will try to create more awareness about fungal biology. We will talk about the life cycle of fungi and explore their ecological functions. We will see how the classification of fungi is carried out and why this is useful in characterising fungal biodiversity. You will also be introduced to a quantitative technique known as rarefaction analysis that allows one to compare species richness in different locations. Another theme we will explore is harnessing fungal biodiversity. We will see how scientists try to identify bioactive compounds from fungi and why fungi might be important for food security. Finally, we will conclude with a discussion of fungal diseases and how they affect agricultural plants.
D3.2 Fungal life cycle and ecology
Reading and interpreting
- eukaryote
- Single-celled or multicellular organism whose cell contains a distinct nucleus surrounded by a membrane.
Let us begin this section with some basic questions. What is a fungus? Broadly, fungi are a group of single-celled or multicellular eukaryotic organisms. The cell walls consist mostly of a complex carbohydrate known as chitin, which is also found in the exoskeleton of insects and crabs.
The hyphae of fungi, which are usually below ground and invisible, can extend over very large areas in some species. By some criteria, the world’s largest organism is a fungal species Armillaria ostoyae, which has spread over 2000 acres in a forest in the United States. For more information, you may view this video.
- hyphae
- A long filamentous, vegetative structure of a fungus that branches underground. A network of hyphae is collectively known as mycelium.
Multicellular fungi form branching structures known as hyphae. These organisms lack chloroplasts and are unable to perform photosynthesis. Most fungi are decomposers, absorbing nutrients from organic waste products and dead organisms. They store excess sugars as glycogen (as animals do) rather than as starch (as plants do).
- Protista
- A kingdom of unicellular or simple multicellular eukaryotic organisms.
Almost all fungi reproduce by means of spores, which are an important part of their life cycle.3 These characteristics of fungi differentiate them from other eukaryotes, including protists, plants and animals.
Figure D3.1a shows a loaf of bread on which mould (a type of fungus) is growing. How did the fungus reach the bread? For a long time, people thought that non-living matter could spontaneously generate life. Today, we know that mould starts growing on bread because of spores.
Life cycle of fungi
Figure D3.1b shows the life cycle of many fungi. Most fungi form spores that can survive harsh environments and can be dispersed by water or wind. When a spore lands on the loaf of bread and there is sufficient moisture, it germinates and forms a network of hyphae. As the hyphae grow, the fungus eventually starts running out of nutrients. At this stage, the fungus forms fruiting bodies containing spores, and the cycle repeats.
- haploid (n)
- An organism or a cell carrying only one set of chromosomes. See also: diploid.
Under some circumstances, hyphae from two different individuals fuse and undergo sexual reproduction. The hyphae are usually haploid. After hyphal fusion, the haploid nuclei fuse to form a diploid nucleus, which then undergoes meiosis. Eventually, the haploid hyphae form spores.

Figure D3.1a Mould growing on bread.
Vincent van Zeijst, Wikimedia commons, CC-BY-SA 4.0.

Figure D3.1b Life cycle of fungi.
Growth and reproduction of organisms is a central aspect of biology. If you wish to study an organism, its life cycle is one of the first things you are likely to investigate. The life cycle of fungus makes it very convenient for use as a laboratory model organism, especially for genetics. The asexual cycle allows a rapid reproduction rate. The haploid state makes it easy to isolate mutants. The sexual cycle, on the other hand, allows one to perform crosses in a manner similar to plant and animal models.
Ecological relationships between fungi and other organisms
Species interact directly with each other in an ecosystem. Fungi form symbiotic (‘living together’) relationships with other species. They are involved in three kinds of symbiotic relationships:
- Commensalism is a symbiotic relationship in which one species benefits and the other neither benefits nor is harmed by the relationship.
- Mutualism is a symbiotic relationship in which both species benefit.
- Parasitism is a symbiotic relationship in which one species is harmed while the other benefits.
The life cycle shown in Figure D3.1b suggests that fungi grow in isolation. Many fungi, however, are closely associated with plant species. Mycorrhizae are symbiotic associations between plants and fungi. The fungi are associated with the plant root system. The fungal hyphae help to extract nutrients such as nitrates and phosphates from the soil, which promote the growth of the host plant. The fungus, in turn, receives carbohydrates from the plant.4
Even more interesting is that fungal hyphae from one individual fungus can connect the roots of many plants, even different species of plants (Figure D3.2). Such a fungus network is called a wood wide web. These hyphal networks allow transport of nutrients between plants.

Figure D3.2 Ecological interactions between fungi and plants.
Mycorrhizal fungi connect roots of different plants. The hyphae transfer carbon between trees.5 The fungi form a network connecting the plants. Mycorrhizal networks communicate dangers, such as pest infestation, to neighbouring, uninfested plants.6 This video provides an overview of the wood wide web.
- endophytic fungi
- Fungi that reside within plant tissues without harming their hosts.
- bioactive compounds
- Substances that influence metabolic processes and can have beneficial impacts on human health.
Another fascinating set of plant–fungal interactions is seen with endophytic fungi which are present in internal tissues of plants. Many medicinal plants are associated with endophytic fungi. The interactions between the plant and its associated fungi influence the production of bioactive compounds and the growth of the plant itself (Figure D3.3b).
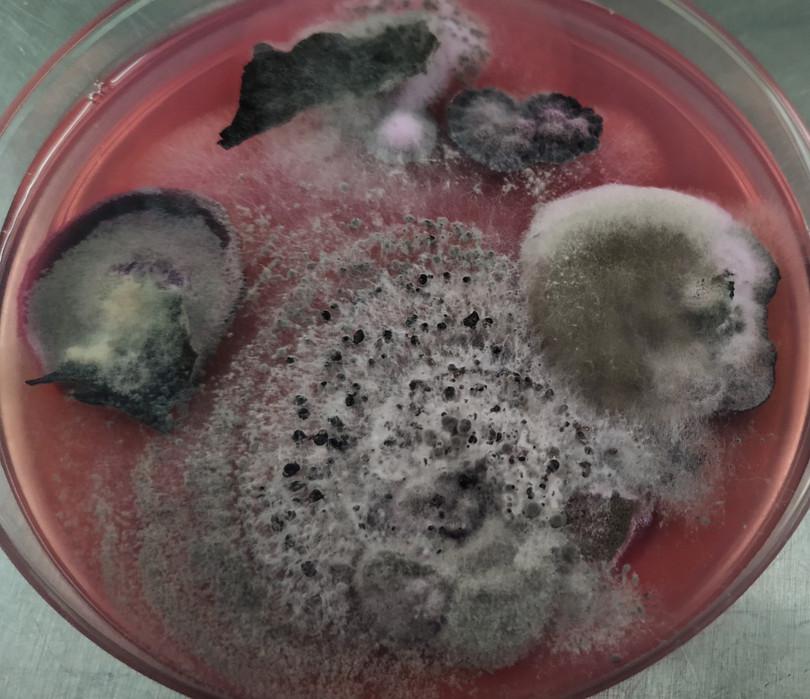
Figure D3.3a Fungi isolated from cold-tolerant trees.
Beena DB.

Figure D3.3b Interactions between endophytic fungi and plants.
The examples we have studied so far have involved terrestrial ecosystems. Fungi are also found in aquatic environments. In marine environments, these fungi are often associated with other organisms such as algae, corals and diatoms (Figure D3.4). Marine fungi are also found in environments such as mangrove forests, where they have a symbiotic relationship with plants and play an important role in nutrient cycling. Some fungal species infect fish and other marine animals and have a parasitic relationship with their host.

Figure D3.4 Ecology of marine fungi.
Adapted from Gladfelter, AS, James, TY and Amend, AS, ‘Marine Fungi’, Current Biology 29, no. 6 (2019): R191–195, doi: 10.1016/j.cub.2019.02.009.
Summary
Fungi are eukaryotic organisms that form branching structures known as hyphae through which they absorb nutrients from their environment. Most fungi form spores that germinate under favourable environmental conditions to give rise to hyphae. These hyphae can participate in both sexual and asexual reproduction. Many fungi are closely associated with plants, and plant–fungus interactions play an important role in nutrient cycling and the production of bioactive compounds. Fungi are also found in marine environments where they may be associated with other marine organisms.
Exercise D3.1 Characterising fungi
Reading and interpretingCopy and complete the table below using information from the text. The first row has been completed for you.
Effect on fungus Effect on host Type of symbiosis Endophytic fungus in plants Shelter and food inside plant cells Induces host plant to produce bioactive compounds Mutualism Mycorrhizal fungi on tree roots Fungi that infect fish Fungi that grow among corals
D3.3 Fungal biodiversity
Scientific process Quantitative skills
Earlier in this chapter, we mentioned that fungi are a diverse set of species and that most of the fungal biodiversity remains undescribed. What do we know about fungal biodiversity in India? Why do scientists investigate fungal biodiversity? Why is it important to document and preserve fungal diversity?
The concept of biodiversity is described in more detail in section A1.3. The importance of taxonomy and systematics is discussed in section C1.3. Section C1.5 describes how scientists use morphological and genetic characteristics for classification of rice varieties. Similar principles apply to the classification of fungal species (though, unlike rice varieties, the individuals being classified belong to different species).
- taxon (pl. taxa)
- A classification level used by taxonomists to group organisms that share defining characteristics.
- genus (pl. genera)
- A taxonomic level that groups different species.
- taxonomy
- The science of naming and classifying all living organisms in a hierarchical manner.
Before we look at India specifically, let us explore fungal biodiversity in general. It is estimated that there are around 3 million species of fungi, but only ~150 000 have been described.2 How can scientists work with such a large number of species? Taxonomists have organised species into higher taxa (genera, families, orders, classes and phyla) based on shared characteristics (some of these characteristics are described below). This classification facilitates identification of species and also allows us to form hypotheses about their life cycle and ecological role. Historically, fungal taxonomy has relied on morphological features. Increasingly, however, molecular techniques based on DNA sequence have become more prominent.
Characteristics of some phyla of fungi
Figure D3.5 highlights some of the morphological features that can be used to identify and classify fungi. Let us explore the figure one panel at a time.

Figure D3.5 Morphological characteristics used to identify fungi.
Adapted from Guarro, J, Gené, J and Stchigel, AM, ‘Developments in Fungal Taxonomy’, Clinical Microbiology Reviews 12, no. 3 (1999): 454–500, doi: 10.1128/CMR.12.3.454.
- coenocytic
- Referring to a cell that has multiple nuclei resulting from nuclear division without cell division.
- aseptate
- Cells that are not separated by walls/septa. See also: septum.
- macroscopic
- Objects that are large enough to be viewed with the naked eye.
- Some defining characteristics of the common black bread mould and similar fungi are shown in the first panel. These fungi reproduce asexually by producing spores inside sporangia. The hyphae are multinucleate and not divided into separate cells (the technical term for this is coenocytic or aseptate). These fungi used to be classified under the phylum Zygomycota, but recently, scientists have argued for a reclassification to better fit the evolutionary history of species in this phylum.7
- The familiar mushrooms that we eat belong to the phylum Basidiomycota. While mushrooms are usually above ground and macroscopic, not all Basidiomycota form mushroom-like structures. Species in this phylum have septated hyphae. In many (but not all) species, some of the septa have hook-like structures known as clamp connections.
- Baker’s yeast and Neurospora crasa (both laboratory model organisms) are members of the phylum Ascomycota. This phylum is differentiated by the formation of a sac-like structure (the ascus) during the sexual cycle. The ascus usually contains eight haploid spores, which are formed after one meiotic and one mitotic division of the diploid nucleus (see sexual cycle in Figure D3.1b).
Identifying many of these characteristics would require a light microscope. Consult section B1.4 if you wish to read more about microscopy.
Fungal biodiversity in India
- mycology
- The study of fungi.
India has a rich history of mycology. The first set of species were described in the latter half of the nineteenth century.8 9 In their 2005 report, Manoharachary et al. state that over 27 000 fungal species have been observed in India.10 Moreover, around 200 genera of fungi were first discovered in India. Almost a third of these new genera were identified and described by one mycologist, CV Subramanian (see extra reading).
Given its long coastline, it is not surprising that marine fungi have been observed in India. According to a report by Borse et al. published in 2013, 207 species of marine fungi have been identified in Indian marine environments. Four fungal species have also been seen in deep sea sediments in the Arabian Sea.11
‘Of the almost 20 000 globally red listed species, all but three are animals and plants. The three fungi consist of two lichens and one mushroom. This gross underrepresentation of fungi on the global Red List greatly hinders the inclusion of fungi in conservation discussions, access to funding programs, policy decisions, and conservation action.’
Conservation of fungal biodiversity does not receive sufficient attention. When you think of endangered species, you might think of birds (see section A1.2) or charismatic mammals such as tigers or lions (see the chapter on felines). However, the International Union for Conservation of Nature (IUCN) maintains a red list of threatened fungal species. For conservation purposes, it is necessary to systematically document fungal biodiversity and how it is being affected by human activity. For example, RK Verma examined observation records of forest fungi over a period of 15 years in central India. He found that 12.3% of the 2700 species that have been reported have only been collected once and appear to be threatened.12
Deuteromycota is no longer considered to be a proper phylum. Species that were classified in this phylum are evolutionarily closer to fungi in other phyla than to each other.
Exercise D3.2 Exploring fungal diversity in India
Quantitative skillsThe following table is taken from Manoharachary et al.10 It shows the number of genera that have been reported in different fungal phyla in India and across the world.
Phylum Number of genera reported India World Myxomycota 380 450 Mastigomycota 205 308 Zygomycota 50 55 Ascomycota 745 2000 Basidiomycota 232 357 Deuteromycota 468 4100 Total 2080 7270 Fungal diversity in India and the world.
Manoharachary, C, Sridhar, K, Singh, R, Adholeya, A, Suryanarayanan, TS, Rawat, S and Johri, BN, ‘Fungal Biodiversity: Distribution, Conservation and Prospecting of Fungi from India’, Current Science 89, no. 1, (2005): 58–71
- How would you depict this data using a graph or visualisation?
- Are all fungal phyla equally diverse? Explain your answer with reference to the table.
- Are some genera overrepresented in India compared to the world? Justify your answer.
Extra Reading Professor CV Subramanian

Photograph of Prof. CV Subramanian.
Bhat, DJ, Muthumary, J, Rajendran, C, RaghuKumar, S and Vittal, BPR, ‘CV Subramanian: Living Legends in Indian Science’, Current Science 106, no. 10 (2014): 1438–1444.
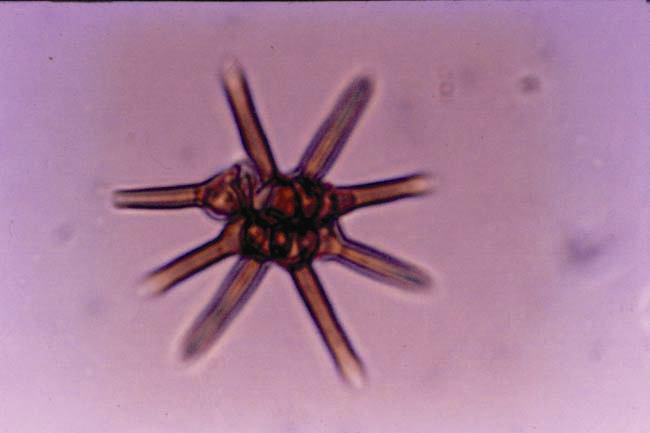
Photograph of Ashtaangam sundaram (eight-armed beautiful fungus in Sanskrit) discovered by CV Subramanian.
Bhat, DJ, Muthumary, J, Rajendran, C, RaghuKumar, S and Vittal, BPR, ‘CV Subramanian: Living Legends in Indian Science’, Current Science 106, no. 10 (2014): 1438–1444.
- monograph
- A document (usually in the form of a book) that is written about a single scholarly topic, usually by a single author.
- deuteromycota
- A division of Fungi in which sexual reproduction has never been observed. This group of fungi is no longer considered to be a valid taxon. Also known as: fungi imperfecti.
Chirayathumadom Subramanian (1924–2016) was a prominent mycologist who was associated with the Centre for Advanced Study in Botany at the University of Madras. He made many seminal contributions to our understanding of the ecology, taxonomy, developmental biology and evolution of fungi. Subramanian discovered many species of fungi, especially hyphomycetes (these fungi were historically classified under the Deuteromycota phylum) and authored a monograph (book) titled Hyphomycetes: Taxonomy and Biology.
Subramanian was known for giving Sanskrit names to genera and species that he discovered (for example, Angulimaya, Ashtaangam and Dwayabeeja). He has left an important legacy for mycology in India by establishing the Mycological Society of India and by starting the journal Kavaka (the Sanskrit name for fungi), which publishes scientific articles on mycology.
Rarefaction analysis
- endophytic fungi
- Fungi that reside within plant tissues without harming their hosts.
We conclude this section by looking at a study carried out by Nalini, Sunayana and Prakash on the diversity of endophytes associated with medicinal plants found in the Western Ghats.13 They wanted to isolate endophytic fungi from various plant tissues (stem, root, rhizome and inflorescence) in seven medicinal plants and compare the diversity of these fungi in the monsoon and winter seasons. One of the techniques they used is rarefaction analysis, which is the focus of this subsection.
Before we examine rarefaction analysis, let us ask a general question. How can we know that there are more endophyte species associated with one plant than another? We could:
- isolate all the fungi present in both plants
- count the number of fungal species we observe
- make a direct comparison.
Such an exhaustive isolation procedure is usually too cumbersome and expensive in practice. It is difficult to even isolate the same number of fungi from two different plants. If, by chance, we isolated more fungi from one plant species, we would expect a greater diversity of fungal species to be present among these isolates. Does that mean that the plant species has greater endophytic species richness? Rarefaction analysis can be used to address this question. We will first develop an intuitive understanding for this technique and then return to the study by Nalini et al.
Using an analogy to understand rarefaction analysis
Consider the following sentence: The quick brown fox jumps over the lazy dog. This has 35 characters (excluding spaces and punctuation). If we scan the letters one at a time (treating both lowercase and uppercase as the same) from the beginning of the sentence, we will find that the first 14 letters (t, h, e, q, u, i, c, k, b, r, o, w, n, f) are all unique. The 15th letter is an o, which has already appeared as part of ‘brown’. By the time we reach the 35th letter (the g of dog), we have encountered all 26 letters in the English alphabet. We can depict this as a graph with the number of unique letters seen versus the number of characters in the sentence (panel A of Figure D3.6). This kind of graph is known as an accumulation curve. Of the first 23 letters, 20 are unique (almost every character is new). However, the 24th to the 28th characters are all repeats.

Figure D3.6 Intuition behind rarefaction analysis.
‘The quick brown fox …’ sentence is especially constructed to contain all 26 letters of the English alphabet using very few repeated ones. Most normal sentences will not have this property. Consider the first sentence of this chapter: ‘The kingdom of fungi comprises a vast set of species with diverse morphologies and life cycles’. It contains 21 of the 26 characters in the alphabet even though it has almost 80 characters. Panel B of Figure D3.6 shows the accumulation curve for this sentence.
Now suppose we only knew the first 20 characters of the special sentence (until the ‘p’ in ‘jumps’) and the first 40 characters of the first sentence of this chapter (until the ‘c’ in ‘species’). Both these portions have 18 unique letters. Is there a way to compare these two portions in a manner that allows us to predict that the ‘quick brown fox’ sentence has more letters?
One way to answer the question is to consider only the first 20 and not the first 40 characters of the longer portion. This ensures that the portions we are comparing have the same size, but the first 20 characters may not be representative of the entire set of 40.
Instead, we can compute the average number of unique characters present in all subsamples of 20 characters chosen from the set of 40 characters. This approach leads to the rarefaction curves shown in Panel C of Figure D3.6. Consider subsamples of different sizes. As the size increases, we expect increasing numbers of unique letters. However, among subsamples of the same size, some might have fewer unique characters than others due to repetition of the same letter. Taking the average of the number of unique letters allows us to get a representative value for all subsamples of that size. Plotting this average against the size of the subsample gives rise to the rarefaction curve.
Applying rarefaction analysis to fungal biodiversity
We can now make the connection between letters in a sentence and fungal biodiversity comparisons. Think of each letter as a fungal isolate. When we begin the isolation process, most observed individuals will belong to different species. As we proceed, we may start seeing individuals from species that we have already recorded.
- species richness
- The number of different species that coexist in a region or community.
We can depict this process graphically using a species accumulation curve, which shows the number of distinct species recorded as a function of the number of individuals observed. If the isolation process is being carried out at multiple sites, it would be difficult to ensure that the same number of individuals are observed in different locations. To compare species richness between multiple sites, we can plot the rarefaction curve. This shows the average number of species present across all subsamples comprising a given number of individuals.
Now that we have an understanding of rarefaction curves, let us revisit the study conducted by Nalini et al. mentioned at the beginning of this subsection. Figure D3.7 shows the rarefaction curves they obtained during isolations performed in the monsoon season. They obtained around 1000 fungal isolates from all seven species of plants. The number of isolates ranged from 314 obtained from Tylophora asthmatica to 22 from Zingiber sp. The rarefaction curves show that species richness also differs across the seven plant species. More endophytic species were isolated from Tylophora asthmatica.
Species richness is not directly related to the number of isolates. For example, while 191 and 172 isolates were obtained from Rubia cordifolia and Phyllanthus amarus respectively, the species richness is higher in Phyllanthus. Rarefaction analysis allowed the study authors to compare endophytic species richness even though the total number of fungal isolates varied from plant to plant.

Figure D3.7 Rarefaction curves of endophytic fungi isolated from medicinal plants.
Adapted from Nalini, MS, Sunayana, N and Prakash, HS, ‘Endophytic Fungal Diversity in Medicinal Plants of Western Ghats, India’, International Journal of Biodiversity (2014): 1–9, doi: 10.1155/2014/494213.
Exercise D3.3 Classifying and comparing diversity of car models
Quantitative skillsScientific processDivide your class or study circle into groups and go to a road or an intersection that has a reasonable amount of car traffic.
- How many different car models do you see over 15 minutes of observation? What characteristics will you use to identify the model?
- Repeat the same procedure at a second location (ideally in a very different part of the town/city). Which location is frequented by a more diverse set of cars (in terms of car models)?
Summary
Morphological and genetic characteristics can be used to classify the large number of fungal species that have been recorded both in India and across the world. Rarefaction analysis is a useful tool for comparing species richness. It involves plotting rarefaction curves, which show the average number of unique species found in all subsamples with a given number of individuals. Conservation of fungi is necessary in view of the important ecological roles they play and the possibility of harnessing fungal biodiversity for our benefit.
D3.4 Harnessing fungal biodiversity
Scientific tools Bridging science, society and the environment
While fungal biology is interesting in its own right, there are some practical reasons for exploring fungal diversity. Many fungi can be used for beneficial purposes. In this section, we will look at two such examples: the isolation of bioactive compounds and the potential of edible mushrooms for food security.
Bioactive compounds
The discovery and development of the antibiotic penicillin was a major milestone in the treatment of bacterial infectious diseases. You probably know how this antibiotic was serendipitously discovered by Alexander Fleming in 1928. He discovered that the mould Penicillium rubens prevented bacteria from growing. Extracts made from P. rubens had antibacterial properties. He named the antibacterial extract ‘penicillin’. The active compound was isolated and identified in 1940.
You may be aware that many of the antibiotics that we use are natural products made by microbes. Many fungi naturally produce bioactive compounds. The pharmaceutical industry is interested in harnessing fungal biodiversity for the discovery of new drugs and other useful molecules such as insecticides.
Given that there are millions of fungal species, how can we identify fungi that may produce a useful compound? And once we isolate such a useful fungus, how can we identify the actual bioactive compound? Let us explore these questions by looking at a couple of studies carried out on endophytic fungi associated with medicinal plants.
Identifying fungi that produce antimicrobial compounds
Mani et al. were interested in identifying endophytic fungi associated with the plant Aegle marmelos (common name bael) that produced antimicrobial compounds. Parts of this plant have a long history of use for treating gastrointestinal issues in Ayurveda, Unani and Siddha systems of medicine. Mani et al. chose to isolate endophytes from this plant due to its medicinal value and prior knowledge that the plant had antimicrobial properties.14
They first screened for fungi producing antimicrobial compounds. Figure D3.8a shows how they carried out this procedure. Each endophyte isolated from the medicinal plant was grown in the centre of several petri dishes. A different pathogenic microbe was also plated in each petri dish. If the fungus produces an antimicrobial compound to that pathogen, the growth of the pathogen is inhibited in the centre of the petri dish.
- zone of inhibition
- The circular area surrounding an antibiotic in which bacterial colonies are inhibited.
- potency
- The amount of drug that would need to be administered in order to obtain a desired effect in the receiver.
In such studies, a control is set up to verify that the pathogen can grow throughout the plate in the absence of the fungus. The control consists of a petri dish without fungus in the centre. By measuring the diameter of the zone of inhibition, the antimicrobial potency of the fungus can be measured. A larger diameter indicates a greater potency.
Mani et al. obtained 169 endophytic isolates from the bael plant. They classified these fungal isolates using morphological methods described earlier. Based on the screen, they observed that five isolates had potent antimicrobial activity. These five strains were identified more precisely using genetic techniques. After this, they grew cultures of these strains and then used organic solvents to extract the metabolites that were secreted. This increases the concentration of bioactive compounds and allows more precise characterisation of the potency of antimicrobial activity.
Identifying bioactive molecules produced by fungi
What can be done after a fungus shows that it can produce bioactive compounds? How do we identify the active molecule? Solvent extraction will not produce a pure compound; it will result in a mixture of metabolites made by the fungus. The next study describes how one bioactive molecule was isolated and identified.
- chromatography
- A technique used to separate components of a mixture by allowing them to flow across a surface.
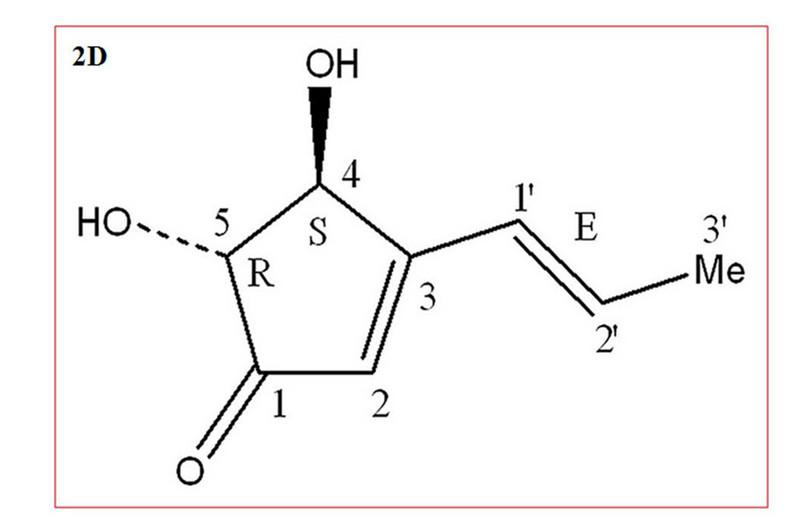
Goutam et al. isolated and identified the active molecule made by the endophytic fungus Aspergillus terreus (which is associated with another medicinal plant). They first separated different compounds present in cultures of this fungus using chromatography. Then they used spectroscopic methods and techniques from organic chemistry (see extra reading) to determine the structure of the active molecule (Figure D3.8c).15
- zone of inhibition
- The circular area surrounding an antibiotic in which bacterial colonies are inhibited.
- potency
- The amount of drug that would need to be administered in order to obtain a desired effect in the receiver.
Extra Reading Chromatography and spectroscopy
Chromatography and other tools from analytical chemistry are very useful in purifying mixtures of compounds. Chromatography relies on separating compounds based on different affinities (attraction) to a particular material (known as the stationary phase). The constituents of the mixture are made mobile by using a gas or a solvent and this mobile phase is passed though the stationary phase. Due to different affinities, the constituents of the mixture move at different speeds and can be separated. For more information about chromatography, you can consult the Khan Academy.
Spectroscopic techniques are also useful in characterising compounds. Different chemical bonds and chemical groups absorb different wavelengths of electromagnetic radiation. By measuring the wavelengths of radiation absorbed by a compound, information about its structure can be obtained.
Another very useful technique for characterising organic compounds is nuclear magnetic resonance (NMR), which is based on how atomic nuclei present in molecules respond to magnetic fields. For more information about spectroscopy and NMR, you may look at this lesson.
Exercise D3.4 Summarising scientific processes
Reading and interpretingScientific process
- Draw a flow chart summarising the steps in the process of identifying and isolating bioactive compounds. Begin with identifying fungi that produce bioactive compounds and end with characterising the bioactive compound.
- Explain why it is necessary to have the control shown in Figure D3.8a.
Edible fungi
Many of us have eaten pizza with mushrooms as a topping. Before mushrooms were commercially cultivated, the only way to obtain mushrooms was to collect wild mushrooms growing in forests. This is risky as some mushrooms are edible, while others can be very poisonous. Adivasi communities who collect food in the forest have a great deal of indigenous knowledge about edible varieties of mushrooms.
A study was carried out by MK Panda and K Tayung in which they documented knowledge of edible mushrooms and their medicinal properties among different adivasi communities of northern Orissa.16 After interviewing 60 people, they documented 19 wild mushroom species that were eaten by adivasis. They also pointed out that 14 of these species were used for medicinal purposes, mostly by traditional healers in the communities.
An important point made by Panda and Tayung is that indigenous knowledge has been transmitted from one generation to the next without any documentation. As different adivasi communities move away from their traditional lifestyles due to loss of forests and other factors, their indigenous knowledge is being lost rapidly. In fact, they observed that knowledge of the medicinal benefits of wild mushrooms lies largely in elder members of the communities.
Why is mushroom cultivation important?
Let us examine why cultivation of mushrooms may play an important role in food security. With a growing population, India faces challenging demands on various resources, including land and water. Feeding the population in a sustainable manner will be an important concern that future generations will need to address.
Mushroom cultivation might be a promising option in this context.17
- Edible mushrooms have excellent nutritional properties and can provide several vitamins and minerals, besides being an inexpensive source of all essential amino acids.
- Mushrooms are usually fast growing and high yields can be obtained using limited resources and on a small scale.
- Mushrooms do not require sunlight for growth, and can be cultivated using agricultural waste with minimal maintenance.
- Unlike most plant-based foods, mushrooms can be grown in a vertical format using shelving, and do not require vast amounts of space.
- Mushroom cultivation provides rural livelihoods, especially for women.18 In China, mushroom cultivation has played a crucial role in poverty reduction and food security.19
Summary
Fungal biodiversity can be harnessed for many beneficial purposes. Many fungi produce bioactive compounds that have antimicrobial properties. Isolating fungi and screening for bioactive compounds is an active area of research. Edible fungi such as mushrooms are also a very promising option for food security. They are nutrient rich and can be grown with minimal resources. Mushroom cultivation is an attractive option for sustainable rural livelihoods.
D3.5 Fungal diseases
Reading and interpreting Bridging science, society and the environment
Like other aspects of fungal biology, diseases caused by fungi do not receive as much attention as they should. Almeida, Rodrigues and Coelho have pointed out that fungal diseases account for nearly 1.6 million deaths annually across the world, and around a sixth of the human population is affected by some severe fungal disease.20 Fungal diseases that affect plants and animals can have severe ecological consequences and some host species may be driven to extinction. Some agricultural crops are prone to fungal infection, which can both directly and indirectly affect human lives and livelihoods.
Cochliobolus miyabeanus and the 1943 Bengal famine
The Bengal famine in 1943 is one of the most tragic events in the history of the Indian subcontinent with around 2.5 million deaths attributed directly or indirectly to that crisis. In the year before the famine, the rice crop in the region was severely affected by the brown spot disease, which is caused by the fungus Cochiliobolus miyabeanus.
According to the mycologist SY Padmanabhan, who was present in Bengal at the peak of the famine, the rice yield of some varieties was reduced by as much as 90%.21 Moreover, weather conditions during that season were very conducive to the fungal outbreak. A cyclone that hit Bengal in October 1942 may have spread fungal spores over a wide region.
While the fungal disease clearly reduced food supply, it is debatable whether it was the sole cause of the famine. Economists such as Amartya Sen have argued that economic factors such as rising food prices (due to the war and war-related policies) and loss of agricultural livelihoods contributed to the famine.
How does the brown spot disease spread? The brown spot fungus can survive in soil and infected plant parts (including seeds and grains) for a few years. The first set of infections usually arises when infected seeds are planted. Thereafter, wind disperses spores from infected plant parts to other plants. The fungus can infect several parts of the plant, including leaves and the panicle (see section C1.3).
Infected parts show visible spots (as shown in Figure D3.9) and these spots continue to grow as the infection progresses. When the spots combine, the leaf wilts.

Figure D3.9 Brown spot disease in a rice plant.
Donald Groth, Wikimedia commons, licensed under CC-BY 3.0.
Brown spot disease remains a concern even today with millions of hectares being affected regularly all over the world. On average, brown spot disease reduces yield by 10% in India and surrounding countries.22 Infections are becoming more common due to more frequent occurrence of drought conditions, which render plants more susceptible to the fungus.
Several control measures are now available, including the use of fungicides and disease resistant rice varieties. Biological control of the disease can be carried out by using fungus from the genus Trichoderma. Trichoderma strains can inhibit the pathogenic fungus by outcompeting it for soil nutrients and by producing bioactive compounds. The Trichoderma strains themselves do not harm the plant.
Fusarium wilt and bananas
After looking at brown spot disease in rice (an important staple crop), let us now consider a fungal disease that threatens one of the most consumed fruits, namely, bananas. Fusarium oxysporum is a soil-borne fungus that infects many plants, including bananas. Infection usually begins when germinating spores or hyphae penetrate the root of the plant. The hyphae then enter the vascular system and spread throughout the plant. Eventually, fungal secretions and growth block the vascular system, leading to wilting of the plant (Figure D3.10).

Figure D3.10 Fusarium wilt in a banana plant.
Scot Nelson, Flickr, public domain.
- cultivar
- A plant variety that is maintained through selective breeding.
Until the 1950s, the Gros Michel cultivar was popular in plantations that were growing bananas for export. However, such plantations were extremely susceptible to Fusarium wilt. As a response to Fusarium wilt, Cavendish cultivars were introduced. Cavendish cultivars were resistant to the strains of Fusarium wilt that were infecting the Gros Michel cultivars.
Around half the bananas grown in the world today are Cavendish cultivars. Unfortunately, in 1967, a strain of Fusarium that could infect Cavendish cultivars was identified in Taiwan.23 Designated TR4, this has been gradually spreading across the world with the first confirmed infections in India reported in 2015. Most recently, infections have been observed in Columbia in 2019. Given its potential for devastation of banana plantations, especially due to monoculture of Cavendish cultivars, the TR4 strain has been called ‘Banana Covid’ in some news media reports.
Figure D3.11 shows some of the ways by which the disease spreads.24 Over short distances, farming activity as well as animal movements and water runoff can spread the disease. Long distance dispersal is usually through human activity.

Figure D3.11 Mechanisms responsible for spreading Fusarium wilt.
Commercially grown banana plants do not form viable seeds and cannot be bred with naturally resistant varieties. There is very little genetic variation within a cultivar because all the plants are produced by asexual reproduction. Given that banana plantations are important for livelihoods and food security, finding ways of controlling TR4 infections is an active area of research.
Soil solarisation is a pre-plantation technique used to control TR4 infection. For a few weeks or months before planting, transparent plastic sheets are placed on top of the soil to heat it using solar radiation. The resulting increase in temperature can inhibit the growth of Fusarium and other pathogens and reduce the chances of propagation of diseases. Note that spores of Fusarium can survive in soil for years.
Summary
Fungal diseases affect animals and plants and remain an understudied class of pathogens. Some plant diseases can spread rapidly due to dispersal of spores either by wind or by human and animal activity. Diseases that affect agricultural crops can have profound economic consequences. Brown spot disease in rice and Fusarium wilt in bananas are examples of fungal diseases.
D3.6 Quiz
Question D3.1 Choose the correct answer(s)
Streptomycetes are bacteria which are typically found in soil. Like fungi, they form branched hyphae and for a long time they were classified as fungi. Which of the following characteristics can be used to distinguish streptomycetes from fungi?
- Both fungi and streptomycetes form spores.
- Only fungi have chitin in their cell walls.
- DNA is the genetic material in both fungi and streptomycetes.
- Streptomycetes do not have a true nucleus. Fungi have a membrane-bound nucleus.
Question D3.2 Choose the correct answer(s)
Which of the following is true of mycorrhizae?
- Mycorrhizae are associated with plant roots.
- Both fungus and plant benefit from mycorrhizae, therefore this is not a commensal relationship.
- Both fungus and plant benefit from mycorrhizae, therefore this is a mutualistic relationship.
- Mycorrhizal fungi obtain sugars from their plant host. These sugars are produced through photosynthesis.
Question D3.3 Choose the correct answer(s)
Which of the following are possible through harnessing fungal biodiversity?
- Edible fungi such as mushrooms are easy to grow and are nutrient-rich.
- Fungi such as those belonging to the genus Trichoderma have been used for biological control of plant pathogens including the one responsible for brown spot on rice.
- Bioactive compounds made by several fungi are actively being screened for pharmaceutical applications.
- Changing food preferences is not a matter of just identifying replacements that can provide the same nutrition. Taste and texture are also important.
Question D3.4 Choose the correct answer(s)
In the table below, the first column indicates names of three fungal phyla and the right column indicates some morphological characteristics.
| Phylum | Morphological characteristics |
|---|---|
| 1. Ascomycota | a. mushroom-like structures |
| 2. Basidiomycota | b. aseptate hyphae |
| 3. Zygomycota | c. a sac-like structure with eight spores |
Identifying fungal phyla.
Which of the following represents the correct association between the phylum and morphological features?
- Mushroom-like structures are characteristic of Basidiomycota.
- Mushroom-like structures are characteristic of Basidiomycota.
- Mushroom-like structures are characteristic of Basidiomycota, ascus containing eight spores (typically) is characteristic of Ascomycota, and aseptate hyphae are characteristic of Zygomycota.
- Mushroom-like structures are characteristic of Basidiomycota.
Question D3.5 Choose the correct answer(s)
The following table is taken from a review article on Fusarium wilt by Thangavelu et al.23 It shows how the TR4 strain of Fusarium oxysporum has spread across the world.
| Country | Year of detection |
|---|---|
| Taiwan | 1967 |
| Philippines | 1970s |
| Indonesia and Malaysia | 1990s |
| Australia | 1997–1999 |
| China | 2001 |
| Oman, Jordan, Lebanon, Pakistan | 2012 |
| Mozambique | 2013 |
| Laos, Myanmar, Vietnam | 2014–2017 |
| India | 2015 |
| Israel | 2016 |
| Colombia | 2019 |
Spread of Fusarium oxysporum.
Thangavelu, R, Loganathan, M, Arthee, R, Prabakaran, M and Uma, S, ‘Fusarium Wilt: A Threat to Banana Cultivation and Its Management.’, CAB Reviews 15, no. 4 (2020): 1–24.
Based on the table, which of the following statements are accurate?
- The TR4 strain has infected banana plants in Australia and Mozambique.
- Between 1960 and 1990, the TR4 strain was found mainly on island states.
- The TR4 strain has spread to Asia, Australia, Africa, South America.
- The TR4 strain has been seen in island states such as Philippines and Indonesia. It is not restricted to land-based transmission.
Question D3.6 Choose the correct answer(s)
The effect of bioactive compounds produced by two different endophytic fungi on two bacterial pathogens is evaluated using the method described in section D3.4. The zones of inhibition obtained are shown in the figure below.

Zones of inhibition of two endophytic fungi.
Based on the figure, which of the following are accurate?
- A zone of inhibition is obtained against both pathogens.
- The growth of pathogen 1 is not inhibited.
- A larger zone of inhibition is obtained against pathogen 2 than pathogen 1.
- Fungal isolate 1 would be a better candidate as it is producing compounds that are effective against more pathogens.
Question D3.7 Choose the correct answer(s)
Before you attempt this question, you should go through Exercise A1.3, which defines species richness and Shannon’s diversity index.
The following table is taken from the article by Nalini et al. described in section D3.3. They were interested in isolating endophytic fungi from various plant tissues (stem, root, rhizome and inflorescence) in seven medicinal plants and comparing the diversity of these fungi in the monsoon and winter seasons. The table below summarises some of their observations.13
| Plant species | Monsoon | Winter | ||||
|---|---|---|---|---|---|---|
| Total isolates | Total species richness | Shannon diversity index | Total isolates | Total species richness | Shannon diversity index | |
| Tylophora asthmatica | 314 | 19 | 2.60 | 143 | 7 | 1.66 |
| Rubia cordifolia | 191 | 9 | 2.05 | 72 | 6 | 1.62 |
| Plumbago zeylanica | 107 | 8 | 1.96 | 63 | 4 | 1.14 |
| Phyllanthus amarus | 172 | 14 | 2.29 | 107 | 11 | 2.27 |
| Eryngium foetidum | 117 | 9 | 1.68 | 47 | 6 | 1.53 |
| Centella asiatica | 123 | 8 | 1.88 | 45 | 6 | 1.57 |
| Zingiber sp. | 22 | 8 | 1.81 | 6 | 2 | 0.64 |
Comparison of isolated endophytic fungi in different seasons.
Nalini, MS, Sunayana, N and Prakash, HS, ‘Endophytic Fungal Diversity in Medicinal Plants of Western Ghats, India’, International Journal of Biodiversity (2014): 1–9, doi: 10.1155/2014/494213.
Based on the table, which of the following statements are accurate?
- Compare species richness in Rubia cordifolia and Phyllanthus amarus during the monsoon season.
- Compare species richness and the Shannon diversity index in Plumbago zeylanica and Eryngium foetidum during the monsoon season.
- Both the species richness and the Shannon diversity index are the highest for Tylophora asthmatica during the monsoon season.
- Look at the numbers for Phyllanthus amarus during the winter season.
Question D3.8 Choose the correct answer(s)
Which of the following graphs could be a rarefaction curve?

Curve types.
- The average number of species should increase (and not decrease) as the number of individuals increases.
- For a small number of individuals, the rarefaction curve should increase at the highest rate (steepest slope) as most individuals will belong to different species.
- This shows a steep increase in the beginning and begins to saturate for larger number of individuals. It is the characteristic shape of a rarefaction curve.
- The rarefaction curve cannot decrease with an increasing number of individuals.
D3.7 References
-
Hawksword, DL, ‘The Magnitude of Fungal Diversity: The 1.5 Million Species Estimate Revisited’, Mycological Research 105, no. 12 (2001): 1422–1432, doi: 10.1017/S0953756201004725. ↩
-
Cheek, M et al., ‘New Scientific Discoveries: Plants and Fungi’, Plants, People, Planet 2, no. 5 (2020): 371–388, doi: 10.1002/ppp3.10148. ↩ ↩2
-
Kendrick, B, Fungi and the History of Mycology (Chichester: eLS, John Wiley, 2011), doi: 10.1002/9780470015902.a0002320.pub2. ↩
-
Van der Heijden, MGA, Martin, FM, Selosse, M-A and Sanders, IR, ‘Mycorrhizal Ecology and Evolution: The Past, the Present, and the Future’, New Phytologist 205, no. 4 (2015): 1406–1423, doi: 10.1111/nph.13288. ↩
-
Simard, SW, Perry, DA, Jones, MD, Myrold, DD, Durall, DM and Molina, R, ‘Net Transfer of Carbon between Ectomycorrhizal Tree Species in the Field’, Nature 388, no. 6642 (1997): 579–582, doi: 10.1038/41557. ↩
-
Song, YY et al., ‘Hijacking Common Mycorrhizal Networks for Herbivore-Induced Defence Signal Transfer between Tomato Plants’, Scientific Reports 4, no. 1 (2014): 3915, doi: 10.1038/srep03915. ↩
-
Spatafora, JW, Chang, Y, Benny, GL, Lazarus, K, Smith, ME and Berbee, ML, ‘A Phylum-Level Phylogenetic Classification of Zygomycete Fungi Based on Genome-Scale Data’, Mycologia 108, no. 5 (2016): 1028–1046, doi: 10.3852/16-042. ↩
-
Manoharachary, C, ‘History of Mycology from India: Some Glimpses’, in Developments in Fungal Biology and Applied Mycology, eds T Satyanarayana, SK Deshmukh and BN Johri, pp. 29–38 (Singapore: Springer Nature, 2017). ↩
-
Subramanian, CV, ‘The Progress and Status of Mycology in India’, Proceedings: Plant Sciences 96, no. 5 (1986): 379–392, doi: 10.1007/BF03053316. ↩
-
Manoharachary, C, Sridhar, K, Singh, R, Adholeya, A, Suryanarayanan, TS, Rawat, S and Johri, BN, ‘Fungal Biodiversity: Distribution, Conservation and Prospecting of Fungi from India’, Current Science 89, no. 1, (2005): 58–71. ↩ ↩2
-
Borse, BD, Borse, KN, Pawar, NS and Tuwar, A, ‘Marine Fungi from India-XII: A Revised Check List’, Indian Journal of Geo-Marine Sciences 42, no. 1 (2013): 110–119. ↩
-
Verma, RK, ‘Biodiversity and Conservation of Forest Fungi of Central India’, in Microbial Diversity and Biotechnology in Food Security, eds RN Kharwar et al., pp. 543–559 (New Delhi: Springer India, 2014), doi: 10.1007/978-81-322-1801-2_49. ↩
-
Nalini, MS, Sunayana, N and Prakash, HS, ‘Endophytic Fungal Diversity in Medicinal Plants of Western Ghats, India’, International Journal of Biodiversity (2014): 1–9, doi: 10.1155/2014/494213. ↩ ↩2
-
Mani, VM, Soundari, APG, Karthiyaini, D and Preeth, K, ‘Bioprospecting Endophytic Fungi and Their Metabolites from Medicinal Tree Aegle Marmelos in Western Ghats, India’, Mycobiology 43, no. 3 (2015): 303–310. doi: 10.5941/MYCO.2015.43.3.303. ↩
-
Goutam, J, Sharma G, Tiwari VK, Mishra A, Kharwar RN, Ramaraj V and Koch B, ‘Isolation and Characterization of “Terrein” an Antimicrobial and Antitumor Compound from Endophytic Fungus Aspergillus terreus (JAS-2) Associated from Achyranthus aspera Varanasi, India’, Frontiers in Microbiology 8 (2017), doi: 10.3389/fmicb.2017.01334. ↩
-
Panda, MK and Tayung, K, ‘Documentation and Ethnomedicinal Knowledge on Wild Edible Mushrooms Among Ethnic Tribes of Northern Odisha, India’, Asian Journal of Pharmaceutical and Clinical Research 8, no. 4 (2015): 139–143. ↩
-
Pandey, VV, Kumari, A, Kumar, M, Saxena, J, Kainthola, C and Pandey, A, ‘Mushroom Cultivation: Substantial Key to Food Security’, Journal of Applied and Natural Science 10, no. 4 (2018): 1325–1331, doi: 10.31018/jans.v10i4.1941. ↩
-
Shahi, V, Shahi, B, Kumar, V, Singh, KM and Kumari, P, ‘Impact Study on Mushroom Cultivation for Micro Entrepreneurship Development and Women Empowerment’, Journal of Pharmacognosy and Phytochemistry 7, no. 4S, (2018): 1–4. ↩
-
Zhang, Y, Geng, W, Shen, Y, Wang, Y and Dai, Y, ‘Edible Mushroom Cultivation for Food Security and Rural Development in China: Bio-Innovation, Technological Dissemination and Marketing’, Sustainability 6, no. 5 (2014): 2961–2973, doi: 10.3390/su6052961. ↩
-
Almeida, F, Rodrigues, ML and Coelho, C, ‘The Still Underestimated Problem of Fungal Diseases Worldwide’, Frontiers in Microbiology 10, no. 214 (2019), doi: 10.3389/fmicb.2019.00214. ↩
-
Padmanabhan, SY, ‘The Great Bengal Famine’, Annual Review of Phytopathology 11, no. 1 (1973): 11–24, doi: 10.1146/annurev.py.11.090173.000303. ↩
-
Barnwal, MK et al., ‘A Review on Crop Losses, Epidemiology and Disease Management of Rice Brown Spot to Identify Research Priorities and Knowledge Gaps’, European Journal of Plant Pathology 136, no. 3 (2013): 443–457, doi: 10.1007/s10658-013-0195-6. ↩
-
Thangavelu, R, Loganathan, M, Arthee, R, Prabakaran, M and Uma, S, ‘Fusarium Wilt: A Threat to Banana Cultivation and Its Management.’, CAB Reviews 15, no. 4 (2020): 1–24. ↩ ↩2
-
Dita, M, Barquero, M, Heck, D, Mizubuti, ESG and Staver, CP, ‘Fusarium Wilt of Banana: Current Knowledge on Epidemiology and Research Needs Toward Sustainable Disease Management’, Frontiers in Plant Science 9 (2018), doi: 10.3389/fpls.2018.01468. ↩